




Classification and Nomenclature of Organic Compounds Containing Halogens
Organic Compounds Containing Halogens are a fundamental class in JEE Main Chemistry, where one or more hydrogen atoms in an organic molecule are replaced by halogen atoms such as fluorine (F), chlorine (Cl), bromine (Br), or iodine (I). These substances include both naturally occurring and synthetic compounds, with wide applications ranging from solvents to pharmaceuticals. Recognising the structure, classification, and reactivity trends of these organohalogen compounds is vital for tackling both conceptual and application-based JEE questions.
Concepts of Organic Compounds Containing Halogens for JEE Main Chemistry
The primary feature of Organic Compounds Containing Halogens is the substitution of hydrogen in an organic skeleton (alkane, arene, etc.) with a halogen atom. The carbon-halogen bond is polar due to the higher electronegativity of halogens compared to carbon. This affects properties like boiling point, reactivity, and mechanism of reactions.
| Type | General Formula | Example |
|---|---|---|
| Alkyl Halides (Haloalkanes) | R–X | Chloroethane (C2H5Cl) |
| Aryl Halides (Haloarenes) | Ar–X | Chlorobenzene (C6H5Cl) |
| Acyl Halides | RCOX | Acetyl chloride (CH3COCl) |
Other related classes include vinyl halides, allyl halides, and benzyl halides. Proper identification of the halogen functional group and its position in structure is essential for both nomenclature and reactivity analysis.
Classification and Nomenclature of Halogenated Organic Compounds
Naming these compounds follows IUPAC rules, where the halogen is treated as a prefix (fluoro-, chloro-, bromo-, iodo-) along with the parent hydrocarbon. Alkyl halides are classified as primary (1°), secondary (2°), or tertiary (3°) based on the nature of the carbon attached to the halogen. Aryl halides have halogen atoms directly bonded to aromatic rings.
- 1-bromopropane (primary alkyl halide)
- 2-chlorobutane (secondary alkyl halide)
- Bromobenzene (aromatic halide)
- Vinyl chloride (halogen on sp2 carbon)
Correct and quick identification using structural features is a scoring area in JEE Main Chemistry. Refer to Naming of Polyfunctional Compounds for practice on IUPAC.
Preparation Methods for Organic Compounds Containing Halogens
These compounds can be prepared by several classic organic reactions:
- Free radical halogenation of alkanes: CH4 + Cl2 (in UV) → CH3Cl + HCl
- Electrophilic aromatic substitution for arenes: Benzene + Br2/FeBr3 → Bromobenzene
- Nucleophilic substitution of alcohols: C2H5OH + HBr → C2H5Br + H2O
- Finkelstein and Swarts reactions for halide exchange
A clear understanding of steps, conditions, and reagents is vital. For in-depth reactions, review Methods of Preparation of Haloalkanes and Haloarenes.
Properties and Key Reactions: SN1, SN2, and Eliminations
The C–X bond in organic halides is polar. Physical properties such as boiling point and density increase with the atomic mass of the halogen. Chemical reactivity depends on whether the halogen is attached to an alkyl or aryl group. Alkyl halides undergo two main types of substitution:
| Mechanism | Key Feature | Order of Reactivity |
|---|---|---|
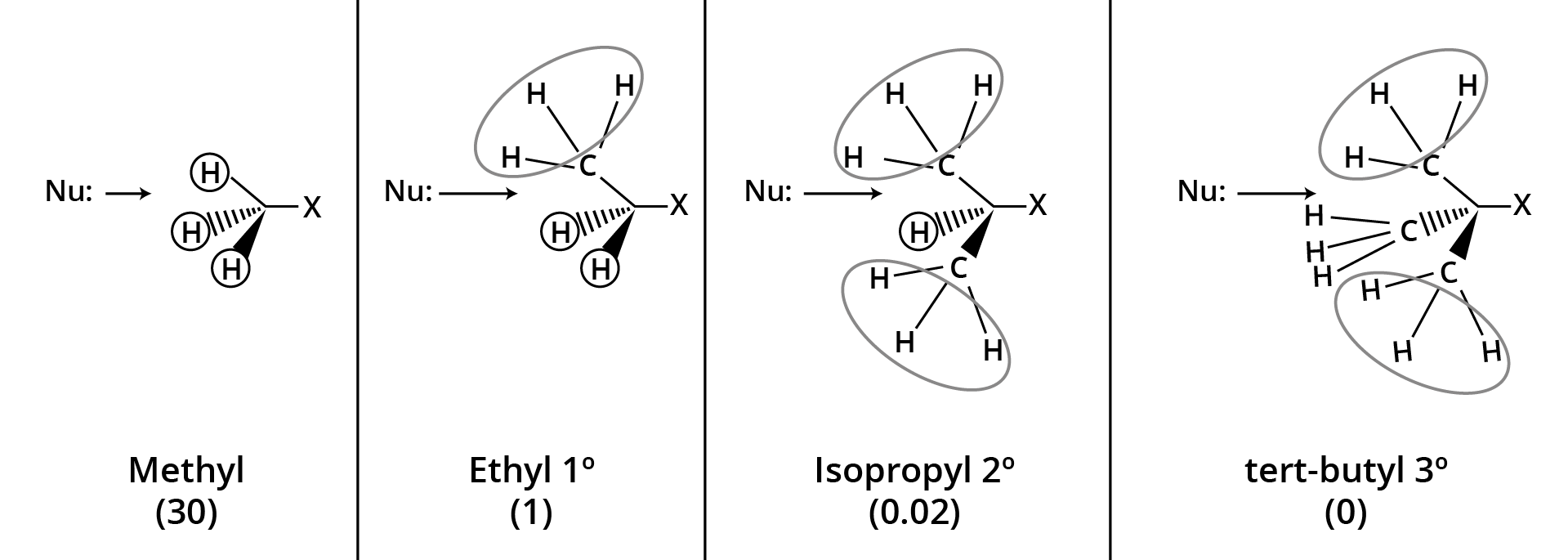
| SN2 (Bimolecular Nucleophilic Substitution) | One-step; inversion of configuration | 1° > 2° > 3° |
| SN1 (Unimolecular Nucleophilic Substitution) | Carbocation intermediate; racemisation | 3° > 2° > 1° |
Elimination (E1/E2) can convert haloalkanes to alkenes. The ease of SN1 or SN2 depends on alkyl structure, leaving group ability, and solvent. For contrast and tips, see Difference Between SN1 and SN2 Reaction.
Noteworthy Examples and Applications
Some crucial halogenated organic compounds for JEE Main include:
| Compound | Formula | Major Use |
|---|---|---|
| Chloroform | CHCl3 | Solvent; former anaesthetic |
| Iodoform | CHI3 | Antiseptic |
| Freons (CFCs) | CCl2F2 | Refrigerants (now phased out) |
| DDT | C14H9Cl5 | Pesticide |
These examples are popular for both MCQs and assertion-reason problems. Application-based scenario questions on the exam may refer to environmental or health impacts—review Environmental Chemistry for context.
Natural vs Synthetic Organohalogens: Environmental Relevance
Most organic halogen compounds are industrially synthesised, but some (e.g., thyroxine, marine alkaloids) occur naturally. Environmental impact is crucial—freons damage the ozone layer, while DDT causes biomagnification. This dimension is increasingly asked in advanced chemistry questions.
- Natural: Thyroxine (hormone, iodine-containing), marine halogenated compounds
- Synthetic: PVC (polyvinyl chloride), chloroform, DDT
- Many are toxic or persistent; safe disposal and alternatives are important topics
Quick Revision Table: Reactions and Key JEE Differences
| Topic | Main Point | Contrast |
|---|---|---|
| Alkyl Halide | Halogen on sp3 carbon | More reactive towards SN2 |
| Aryl Halide | Halogen on aromatic ring | Resistant to nucleophilic substitution |
| SN1 | Carbocation forms | Faster for tertiary |
| SN2 | One-step inversion | Faster for primary |
For mastering substitution and elimination mechanisms, reference SN1 and SN2 Reactions and Nucleophilic Substitution Reaction.
Important JEE Main Study and Practice Links
- Organic Compounds Containing Halogens Mock Test
- Practice Paper on Organohalogen Compounds
- JEE Level Important Questions
- Quick Revision Notes (Vedantu)
- Preparation of Haloarene
- SN1 vs SN2 Difference
- More on Alkyl Halides
- Organic Compounds Containing Oxygen
- Hydrocarbons Chapter Reference
This chapter provides the backbone for understanding substitution and elimination mechanisms in the JEE syllabus. Use links for targeted revision and practice. For further environmental aspects, see Environmental Chemistry.
Vedantu’s experts recommend regular practice of mechanism and application-type questions for Organic Compounds Containing Halogens for JEE Main. Revisit key formulae and mnemonics before attempting mixed-topic mock tests, focusing on both theoretical reasoning and problem-solving speed. Mastering this topic boosts accuracy in functional group identification, reaction prediction, and application-centric questions throughout organic chemistry.
FAQs on Organic Compounds Containing Halogens: Concepts, Examples, and Uses
1. What are organic compounds containing halogens?
Organic compounds containing halogens are compounds where one or more hydrogen atoms in an organic molecule are replaced by halogen atoms like fluorine (F), chlorine (Cl), bromine (Br), or iodine (I).
Key features include:
- They are also called organohalogen compounds or halogenated organic compounds.
- Main types: alkyl halides (haloalkanes) and aryl halides (haloarenes).
- Common examples: chloroform, DDT, chlorobenzene.
- Uses include medicines, solvents, refrigerants, and pesticides.
2. Which chapter covers organic compounds containing halogens for class 12?
Organic compounds containing halogens are typically covered in the chapter "Haloalkanes and Haloarenes" in Class 12 Chemistry (CBSE/NCERT syllabus).
Main points include:
- Classification of haloalkanes (alkyl halides) and haloarenes (aryl halides).
- Nomenclature, preparation, properties, and uses.
- Frequently asked in JEE, NEET, and board exams under organic chemistry chapters.
3. What are halogenated organic compounds?
Halogenated organic compounds are organic molecules in which one or more hydrogen atoms have been substituted with a halogen atom (F, Cl, Br, I).
Key characteristics:
- Classified as alkyl halides (aliphatic chain) and aryl halides (aromatic ring).
- Show unique physical and chemical properties due to the electronegativity of the halogen.
- Include solvents, anesthetics, insecticides, and industrial chemicals.
4. Can you list some examples of halogenated organic compounds?
Common halogenated organic compounds include:
- Chloroform (CHCl3)
- Carbon tetrachloride (CCl4)
- Chlorobenzene (C6H5Cl)
- DDT (Dichlorodiphenyltrichloroethane)
- Bromoethane (C2H5Br)
- Freons (CFCs, e.g., CF2Cl2)
5. Are there natural halogen-containing organic compounds?
Yes, some halogenated organic compounds occur naturally. Examples include:
- Thyroxine (an essential iodine-containing hormone in humans)
- Chloromethane produced by certain plants and algae
- Halogenated alkaloids from marine organisms
6. How are organic compounds containing halogens prepared?
Preparation of organohalogen compounds involves different chemical methods. Key methods include:
- Halogenation of alkanes/alkenes (free radical or electrophilic addition reactions)
- Nucleophilic substitution of alcohols with halogen acids (HX), PCl3, PBr3, etc.
- Sandmeyer reaction (for aryl halides from diazonium salts)
- Industrial synthesis (direct chlorination, bromination, or hydrohalogenation)
7. What is the importance of halogenated compounds in daily life?
Halogenated organic compounds are vital in everyday life and industry due to their unique properties.
- Used as medicines (e.g., halothane anesthetic)
- Function as solvents and cleaning agents (e.g., trichloroethylene)
- Important in refrigerants (e.g., Freons) and pesticides (e.g., DDT)
- Serve as intermediates in chemical synthesis
8. How do you identify alkyl halides and aryl halides?
You can identify alkyl halides and aryl halides by their structure and the position of the halogen atom:
- Alkyl halides (haloalkanes): Halogen attached to an aliphatic (non-aromatic) carbon chain. E.g., ethyl chloride (C2H5Cl).
- Aryl halides (haloarenes): Halogen attached directly to an aromatic ring. E.g., chlorobenzene (C6H5Cl).
9. Why do halogenated organic compounds have higher boiling points?
Halogenated organic compounds often have higher boiling points compared to their non-halogenated counterparts. This is due to:
- Increased molecular weight from the halogen atoms
- Greater dipole-dipole and van der Waals forces caused by the high electronegativity of halogens
- Heavier halogens (Br, I) increase boiling point more than lighter ones (F, Cl)
10. What are the main reactions of organic compounds containing halogens?
Key reactions of organohalogen compounds include:
- Nucleophilic substitution reactions (SN1 and SN2 mechanisms)
- Elimination reactions forming alkenes
- Reactivity trend: Alkyl halides are more reactive than aryl halides in substitution reactions
- Formation of Grignard reagents (RMgX) from alkyl/aryl halides
11. What are the environmental impacts of halogenated organic compounds?
Some halogenated organic compounds can have harmful environmental effects:
- Chlorofluorocarbons (CFCs) contribute to ozone depletion
- DDT and similar pesticides persist in the environment and may disrupt ecosystems
- However, not all are hazardous—some are biologically important or used safely in medicine























