




Introduction to Electrochemistry
Electrochemistry is a sub-field of chemistry related to the study of the relationship between electrical energy and chemical changes. In galvanic cells, when two electrodes are immersed in their respective ions, one electrode (anode) tends to oxidize and the ion on the other electrode (cathode) tends to accept electrons. This tendency to lose electrons (oxidation) or acquire electrons (reduction) is called the electrode potential.
The tendency to lose electrons is measured with the help of oxidation potential.
What is Oxidation Potential?
We can understand the term in a better way by taking an example. Suppose we dipped an electrode in an electrolyte solution containing its ion. This would lead to the electrode releasing its ions into the solution, leaving the electrons in the electrode itself. Thereby a negative charge will be created on the electrode.
It can also accept the positive ions from the solution (or say giving out the electrons) and create a positive charge on it. Thus, there arises a potential difference between the electrode and the solution which is called the electrode potential.
The tendency of these processes to take place is not the same for an electrode. If the effect of releasing the positive ions into the solution is greater than accepting ions from the solution, then the electrode will gain a negative charge on it. Thus, the magnitude of this effect depends on its tendency to lose or gain electrons.
When the metal of an electrode loses its electrons, the electrons remain on the electrode while the positive ions of metal formed pass into the solution. This process is oxidation and can be represented as follows:
$M \rightarrow M^{n+}+n e^{-}$
Thus, the electrode will get a negative charge and will be termed as anode. The potential for this process is called oxidation potential.
Whereas, the potential for reverse process, that is when metal ion in solution takes the electron from the metal, is called reduction potential. Electrode in this case is termed as cathode (have positive charge).
$M^{n +}+n e^{-} \rightarrow M$
Standard Oxidation Potential
It is not possible to calculate the absolute value of potential of a single electrode by just dipping it into an electrolyte directly. However, the difference in potential between two electrodes can be calculated experimentally. So, it is necessary to couple this electrode whose potential has to be calculated with another electrode whose potential is known. This electrode is called a reference electrode.
According to IUPAC convention, reduction potential alone can be considered as electrode potential. This potential at standard condition, that is with 1 molar solution at 298 K is called standard reduction potential. The magnitude for standard oxidation potential will be the same as the reduction potential, only the sign will change since oxidation is just the reverse process of reduction.
Thus, when we define oxidation potential or standard oxidation potential, it is necessary to understand the term reduction potential first.
Need of Analyzing Oxidation Potential of a Metal.
We know that oxidation potential is the potential for losing an electron. Thus, the metal which has greater tendency to lose its electron will have the highest oxidation potential.
For example: if we compare the metal sodium with zinc, Na has only one electron in its outermost shell and can easily release its electron. Thus, sodium will have a higher oxidation potential than Zn.
From this concept, the strength of oxidizing agent and reducing agent can be found out.
The electrochemical series showing standard reduction potential of certain elements is given below:
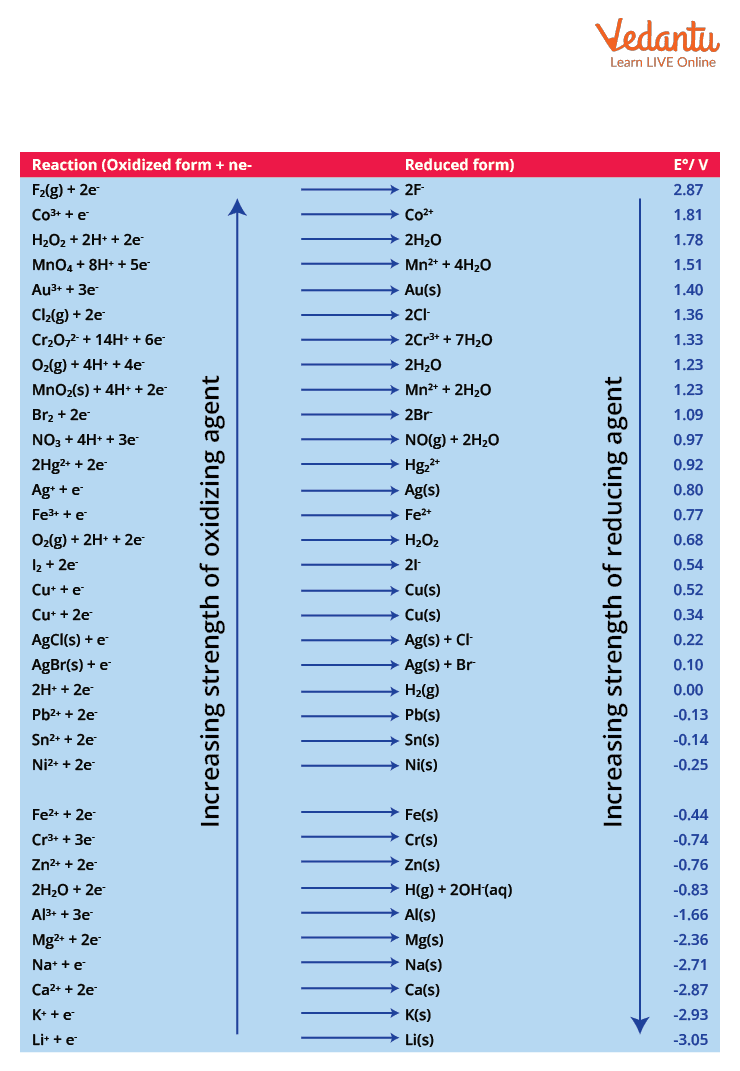
Electrochemical Series Showing the Standard Potential for Certain Electrode Reactions.
What is the Oxidation Number?
Oxidation number is a number given to an element in a compound, which denotes the net number of electrons lost or gained by that element in that particular compound.
For example: to calculate the oxidation number of Mn in KMnO4, we have to identify the net number of electrons lost or gained by Mn. In this compound, the general oxidation number for oxygen and potassium is already known (constant in almost every compound) i.e. -2 and +1 respectively. Since the whole compound is neutral, the sum of charges of all elements in this compound will be equal to zero. Thus:
$\begin{align} &(+1)+M n+(4\times(-2))=0 \\ &+1+M n-8=0 \\ &M n=8-1 \\ &=7 \end{align}$
Important Points to Remember while Calculating Oxidation Number
Oxidation number of an atom in its free elemental state is zero.
Fluorine, which is most electronegative in nature, has oxidation number -1 in all its compounds.
Oxidation number of O in all its compounds is -2. However, in peroxides (O22-), it is -1, in superoxides (O2-) it has $\dfrac{-1}{2}$ and in OF2 oxygen has +2 oxidation number.
Oxidation number of hydrogen in almost every compound is +1. However, in metal hydrides like NaH, BaH2, etc. it is -1.
Oxidation number of all alkali metal elements is +1 and all alkaline earth metal elements is +2.
The net sum of oxidation number of all the atoms in a compound or molecule will be equal to the charge on that molecule.
Conclusion
Almost every reaction involves giving out the electrons or taking it from another species. Thus, it is very important to know the concept of oxidation potential and number in entire chemistry. However reduction potential is used everywhere according to IUPAC convention instead of oxidation potential.
Oxidation and reduction potential can also be called redox potential together for a process which involves both loss and gain of electrons and it is measured in volts or millivolts. Moreover, the term is very crucial while studying electrochemical cells, which involves movement of ions or electrons as a charge carrier.
FAQs on Oxidation Potential and Number for JEE
1. How does pH affect reduction potential?
If the reaction involves either H+ or OH- , its concentration can affect the reduction potential. Since the concentration variation of either of these ions can vary, the equilibrium to forward or backward depends on the reaction’s condition.
We know that reduction potential is the potential for reduction to take place. Thus, if the reaction involves either H+ or OH- , then the variation in concentration can also interfere in the ease of process of reduction reaction in that system. Thus, it will change the reduction potential also.
2. Which is the strongest reducing agent?
Reducing agent is the species that gets oxidized itself and reduces the other species in a reaction. Therefore, the strongest reducing agent should have a high tendency to undergo oxidation. Thus, it will have a highly positive oxidation potential and least standard reduction potential (more negative).
Thus, from the electrochemical series, it can be observed that Li has a standard reduction potential of -3.05 V (oxidation potential will be +3.05 V). Thus, it will be the strongest reducing agent.
























