




How Are Haloarenes Prepared? Main Methods with Equations and Tips
The Preparation of Haloarene is a central topic in JEE Main Organic Chemistry, as it focuses on methods for synthesizing aromatic compounds with halogen substituents (haloarenes or aryl halides). Aromatic halides serve as building blocks in pharmaceuticals, pesticides, and polymers, and understanding these conversions enables students to solve key application-based problems.
A haloarene is an aromatic compound in which a halogen atom (Cl, Br, I, F) directly bonds to an aromatic ring, such as benzene or its derivatives. The general formula is Ar–X, where Ar is the aryl group and X is halogen.
Overview: General Methods of Preparation of Haloarenes
For JEE, typical preparation methods of haloarenes include electrophilic halogenation, diazonium salt substitution (Sandmeyer and Gattermann reactions), and limited direct halogenation from phenol. Each follows specific reagents, conditions, and mechanisms:
- Halogenation of aromatic rings (usually using Cl2/Br2 with a Lewis acid catalyst)
- Substitution via diazonium salts (Sandmeyer/Gattermann reactions)
- From phenol (reaction with halogen acids under specific conditions)
Halogenation of Arenes: Direct Method
The most straightforward way to obtain haloarenes is direct halogenation of benzene (or other arenes), using a halogen (Cl2, Br2) and a Lewis acid catalyst like FeCl3 or AlCl3. The reaction proceeds via electrophilic aromatic substitution.
- Benzene + Cl2 (with FeCl3): Forms chlorobenzene.
- This method does not work for iodination (due to low reactivity) or fluorination (due to high reactivity, risk of side products).
- Limitation: If the ring is already substituted or deactivated, yields drop and side reactions may occur.
Representative equation:
C6H6 + Cl2 (FeCl3) → C6H5Cl + HCl
Preparation of Haloarene via Diazonium Salts: The Sandmeyer Reaction
For preparing haloarenes not accessible by direct halogenation (e.g., chlorobenzene, bromobenzene from aniline), the Sandmeyer reaction is widely used. This route involves converting a primary aromatic amine to a diazonium salt (by reaction with NaNO2 + HCl at 273–278 K), then treating it with CuCl or CuBr to introduce the halogen substituent.
Example of Sandmeyer Reaction:
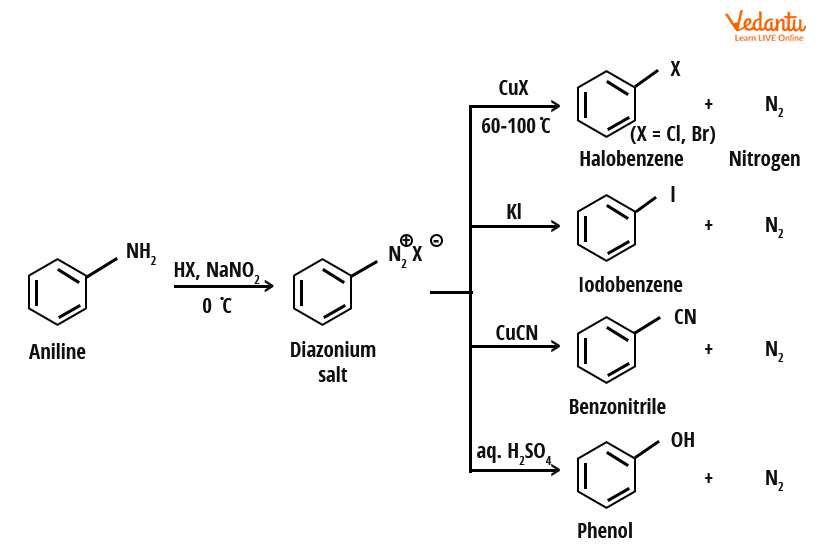
The Sandmeyer reaction is most effective for introducing Cl, Br, or CN groups into the aromatic ring. For iodination, diazonium salt is directly treated with KI (without copper salt).
- Formation of diazonium salt: Aniline + NaNO2 + HCl (cold) → Benzene diazonium chloride
- Substitution with halide: Benzene diazonium chloride + CuCl/CuBr → Chlorobenzene/Bromobenzene
- Free radical mechanism (important for mechanism-based MCQs)
Limitations: Only applicable for aromatic primary amines; diazonium salts must be used immediately as they are unstable above 5 °C.
Mechanism Steps in Sandmeyer Reaction
This sequence is crucial for JEE mechanism questions:
- Nitrosonium ion (NO+) is generated by sodium nitrite and HCl.
- It reacts with the amine to form benzene diazonium ion.
- Copper(I) halide reduces the diazonium ion to form an aryl radical, which finally couples with halide to yield haloarene.
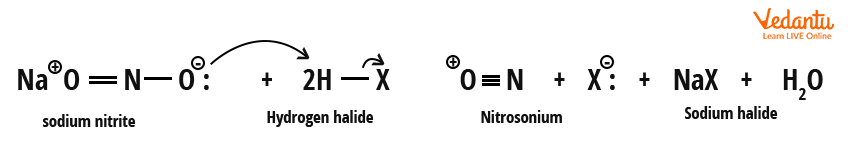
Pay special attention to nitrosonium ion and diazonium ion formation steps; these are commonly tested. The formation of diazonium salt is called diazotization.
Gattermann Reaction: Alternative to Sandmeyer
The Gattermann reaction is an alternative where benzene diazonium salt is treated with Cu powder and halogen acid (HCl or HBr), producing haloarene. It closely resembles Sandmeyer, but uses different reagents. For JEE, recognize that both reactions transform diazonium salts to aryl halides; method choice depends on available reagents.
- Gattermann: Benzene diazonium chloride + Cu/HCl → Chlorobenzene
- Sandmeyer: Benzene diazonium chloride + CuCl → Chlorobenzene
Preparation of Haloarene from Phenol
Conversion of phenol to haloarene involves reaction with halogen acid (HX) and catalyst (like ZnCl2). However, due to the resonance stabilization of phenol and partial double bond character of C–O bond, phenol resists direct halogen substitution under normal conditions. Thus, this method is rarely used outside theoretical discussions, except for iodobenzene with concentrated HI.
- Phenol + HCl + ZnCl2 (Lucas reagent) → Chlorobenzene (low yield, not industrially practical)
- Limited use; important for highlighting differences from haloalkane preparation.
Comparison: Preparation of Haloarenes vs. Haloalkanes
| Aspect | Haloalkanes | Haloarenes |
|---|---|---|
| Common Preparation Route | From alcohols by substitution with HX or PX3 | Electrophilic halogenation, or via diazonium salts |
| Direct from Phenol? | Yes, with HX (Lucas) | No, due to resonance stabilization |
| Reactivity | More reactive to nucleophilic substitution | Resistant to nucleophilic substitution |
| Bond Character | C–X is single bond | C–X has partial double bond character |
| JEE Focus | Properties, SN1/SN2 mechanisms | Sandmeyer, EAS, resonance exceptions |
Read more on methods of preparation of haloalkanes and haloarenes and see Industrial contrasts for JEE clarity.
Practice Problems on Preparation of Haloarene for JEE
- State the limitations of direct halogenation for arene derivatives.
- Explain why phenol fails to yield chlorobenzene using ZnCl2/HCl (Lucas reagent).
- Draw the mechanism for the Sandmeyer reaction starting from aniline.
- Differentiate between Sandmeyer and Gattermann reactions.
- Predict the product: Nitrobenzene → aniline → diazonium salt → KI.
For more MCQs and practice on ORGANIC COMPOUNDS CONTAINING HALOGENS, visit the JEE Main Organic Compounds Containing Halogens Mock Test on Vedantu’s Chemistry section.
Quick Revision Table: Preparation of Haloarene
| Method | Starting Material | Key Reagents/Conditions | Product |
|---|---|---|---|
| Halogenation | Benzene | Cl2/Br2, FeCl3 or AlCl3 | Chlorobenzene/Bromobenzene |
| Sandmeyer Reaction | Diazonium salt (from aniline) | NaNO2, HCl (cold), CuCl/CuBr/CuCN | Chloro-, bromo-, cyanobenzene |
| Gattermann | Diazonium salt | Cu powder, HCl/HBr | Chloro-/bromobenzene |
| From Phenol | Phenol | HX, ZnCl2 (Lucas) | Low yield haloarene |
For deeper conceptual revision, access haloarene revision notes and solved examples on the Vedantu JEE Chemistry portal. Use the table above as a one-glance exam aid.
In summary, the Preparation of Haloarene covers essential JEE reactions: electrophilic aromatic substitution, diazonium chemistry, and important comparisons to haloalkanes. Remember to practice conversions, highlight exceptions, and thoroughly review Sandmeyer mechanism steps for full exam confidence.
FAQs on Preparation of Haloarene: Stepwise Methods and Key Reactions
1. What are the methods of preparation of haloarenes?
Haloarenes can be prepared by three main methods, each commonly asked in exams like JEE or Class 12 boards:
- Direct halogenation of arenes (e.g., chlorination or bromination of benzene using halogen and a Lewis acid catalyst like FeCl3).
- Sandmeyer reaction (conversion of aryl diazonium salt to haloarene using CuX, where X = Cl, Br, or CN).
- From phenol (treating phenol with halogen acids or PCl5/SOCl2).
2. What is the Sandmeyer reaction in haloarene preparation?
The Sandmeyer reaction is an important method for synthesising haloarenes from aromatic amines via diazonium salts.
- It involves converting a primary aromatic amine (like aniline) to a diazonium salt.
- The diazonium salt then reacts with Copper(I) halides (CuCl or CuBr) to form haloarenes.
- This allows introduction of Cl, Br, or CN group to the benzene ring efficiently.
3. How are haloarenes prepared from benzene?
Haloarenes are prepared from benzene mainly by electrophilic aromatic substitution (halogenation):
- Mix benzene with a suitable halogen (Cl2 or Br2).
- Add a Lewis acid catalyst such as FeCl3 or FeBr3 to activate the halogen.
- Monohalogenation yields chlorobenzene or bromobenzene.
4. How is phenol used to prepare haloarenes?
Phenol can be converted to haloarenes using halogen acids or halogenating reagents:
- Treat phenol with PCl5, PCl3, or SOCl2 to obtain chlorobenzene.
- Direct halogenation of phenol in the presence of water can also yield polyhalogenated phenols.
- This method is less common for monohalogenated products due to low selectivity and side reactions.
5. What are the main differences between haloalkanes and haloarenes?
Haloalkanes and haloarenes differ in structure, method of preparation, and chemical properties:
- Haloalkanes contain halogen attached to an sp3-hybridized carbon, while haloarenes have halogen bonded to an sp2-hybridized benzene ring.
- Preparation of haloalkanes is often from alcohols or alkanes, while haloarenes are usually prepared by halogenation, Sandmeyer reaction, or from phenol.
- Haloarenes show lower reactivity towards nucleophilic substitution than haloalkanes due to resonance effects in the benzene ring.
6. What is the preparation method of haloalkanes?
Haloalkanes are commonly prepared by:
- Halogenation of alkanes (with UV light for free radical substitution).
- From alcohols using reagents like PCl5, SOCl2, or HX (nucleophilic substitution).
- Wurtz reaction (for higher alkanes).
7. Why is the Sandmeyer reaction essential for synthesizing certain haloarenes?
The Sandmeyer reaction is essential because some haloarenes (especially iodoarenes and those with electron-withdrawing groups) cannot be made easily by direct halogenation.
- It enables selective introduction of halogens onto the benzene ring through diazonium salt intermediates.
- It helps avoid multiple substitution and enables preparation of chloro, bromo, or cyano derivatives efficiently.
8. Can all arenes be halogenated directly to give haloarenes?
No, not all arenes can be halogenated directly.
- Direct halogenation works well for benzene and simple alkylbenzenes but fails for deactivated rings (such as those with strong electron-withdrawing groups).
- Iodination and fluorination of benzene are difficult and require special conditions.
9. What common mistakes do students make while writing the mechanism for the Sandmeyer reaction?
Common mistakes in the Sandmeyer reaction mechanism include:
- Omitting formation or correct structure of diazonium salt.
- Not showing the role of copper(I) salt (CuCl or CuBr).
- Confusing Sandmeyer with Gattermann reaction (which uses Cu powder and HX).
- Ignoring gaseous by-products such as N2.
10. How do examiners test exceptions and limitations in haloarene preparation reactions?
Examiners often ask application-based or reasoning questions focusing on:
- Chemical limitations, such as why phenol chlorination yields polyhalogenated products, or why direct halogenation fails for deactivated rings.
- Distinguishing between conditions for Sandmeyer vs direct halogenation.
- Predicting or explaining the outcome when unusual substrates or reagents are used.























