




What is Reduction Reaction?
A redox reaction is a chemical reaction in which oxidation and reduction take place simultaneously. These two processes are considered opposite to each other. Reduction reactions are half reactions in which a chemical species gains one or more electrons. The reduction can also be defined as the addition of hydrogen or the removal of oxygen atoms from the reacting species. Reducing agents are those compounds that reduce the chemical compound by giving electrons or protons.
Reduction of Aldehydes and Ketones
Both aldehyde and ketone carbonyl groups can be reduced to hydrocarbons or methylene groups (-CH2-). In addition, aldehydes can be reduced to primary alcohols and ketones can be reduced to secondary alcohols.
Reduction of Aldehyde and Ketone into Hydrocarbon
A very important kind of reduction useful in synthetic Organic Chemistry is the conversion of the carbonyl group (>C=O-) to the methylene group (-CH2-). There are two very popular methods to accomplish this conversion:
(i) The Clemmensen Reduction
(ii) The Wolff-Kishner Reduction
Clemmensen Reduction
Clemmensen reduction was developed by the German chemist E.C. Lemmensen in 1912. When an aldehyde or ketone is heated with zinc amalgam (zinc dissolved in mercury) and concentrated HCl, the carbonyl group is reduced to a methylene group, and the corresponding hydrocarbon is obtained. This reaction is known as Clemmensen reduction. The general reaction is as follows:
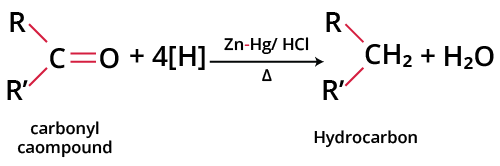
Clemmensen Reduction
For example: Clemmensen reduction of propanal gives propane.

Clemmensen Reduction
The reaction is thought to involve the protonation of oxygen in the carbonyl group and the attack of the electron-deficient carbon by a zinc atom.
Wolff-Kishner Reduction
Wolff-Kishner reduction was developed by Ludwig Wolff and Nikolai Kishner in 1911. When an aldehyde or ketone is heated with hydrazine, followed by heating with a strong base such as KOH or potassium tert-butoxide at 140-200⁰C in a high boiling solvent such as diethylene glycol or dimethyl sulphoxide, the >C=O group is reduced to >CH2 and the corresponding hydrocarbon is obtained. This reaction is called the Wolff-Kishner reduction. The reaction takes place via the formation of hydrazones of the carbonyl compound. The general reaction is as follows:
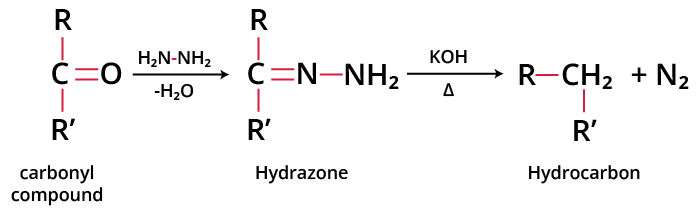
Wolff-Kishner Reduction of Carbonyl Compounds
For example: Benzaldehyde undergoes Wolff-Kishner reduction to produce toluene.

Wolff-Kishner Reduction of Benzaldehyde
Acetone undergoes Wolff-Kishner reduction to produce propane.
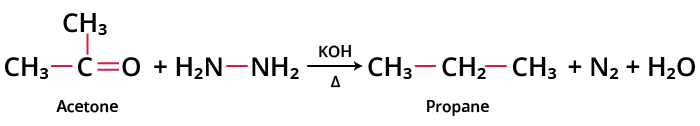
Wolff-Kishner Reduction of Acetone
Reduction of Aldehydes and Ketones to Alcohols
(i) Catalytic Reduction
Reduction by molecular hydrogen in the presence of catalysts like Ni, Pd, Pt, etc. converts aldehydes and ketones into the corresponding alcohols. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohols.
The general reaction is given as:

Catalytic Reduction of Aldehyde to Alcohol and Ketone to Alcohol
For example: On catalytic reduction, acetaldehyde gives ethanol and acetone gives isopropyl alcohol.
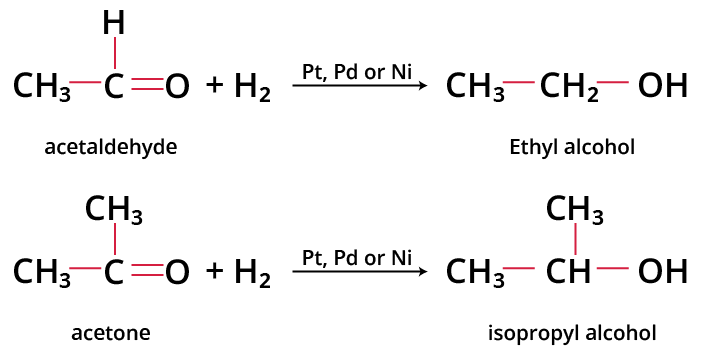
Image: Hydrogenation of Aldehydes and Ketones
(ii) Reduction Using Metal Hydrides
The reaction of an aldehyde or a ketone with sodium borohydride (NaBH4) or lithium aluminium hydride (LiAlH4), followed by water or some other proton source, yields alcohol. Aldehydes are reduced to 1o alcohol and ketones are reduced to 2o alcohol. These reactions involve the irreversible nucleophilic addition of the hydride ion to the carbonyl carbon.
Reducing Agents
The reduction of aldehydes and ketones to alcohol requires a suitable reducing agent. The reducing agents used were lithium aluminium hydride (LiAlH4) and sodium borohydride (NaBH4). Both of the reagents result in the formation of the same end product, irrespective of the reagents used. Of these two reagents, lithium aluminium hydride is more reactive than sodium borohydride.
Thus, LiAlH4 can react violently with solvents like alcohol and water. Therefore, these solvents should be avoided in the process. The reaction can take place at room temperature in the presence of dry ether. Since sodium borohydride is less reactive than LiAlH4, it can be used with alcohol and water in alkaline conditions.
The general reaction is as follows:
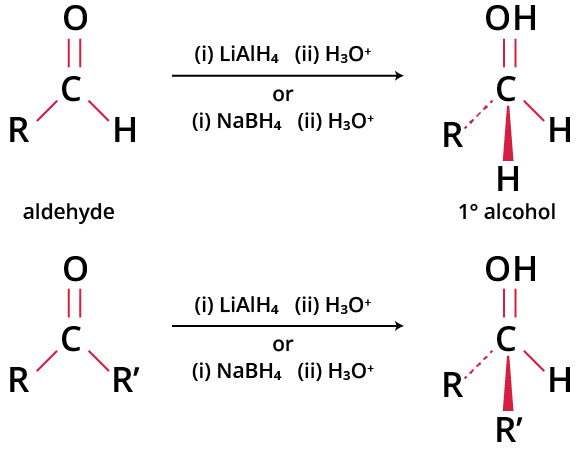
Reduction Reactions of Carbonyl Compounds
However, as LiAlH4 is a very potent reductant, it may sometimes reduce other reducible groups like >C=C< and -C≡C-, particularly if they are conjugated with the carbonyl group. So, selective reduction of the carbonyl group may not be possible with LiAlH4 under such conditions. However, as sodium borohydride is less reactive, it performs a selective reduction of the carbonyl group in a carbonyl compound, leaving the carbon-carbon multiple bonds intact in the product.
(iii) Reduction using Aluminium Isopropoxide in Isopropyl Alcohol Meerwein-Ponndorf-Verley Reduction or MPV Reduction
When heated with aluminium isopropoxide in isopropyl alcohol (propan-2-ol), aldehydes are reduced to their corresponding primary alcohols while ketones are reduced to the corresponding secondary alcohols. This reaction is called Meerwein-Ponndorf-Verley reduction or MPV reduction. It is a reversible reaction known as Oppenauer oxidation.
The general reaction is given as:
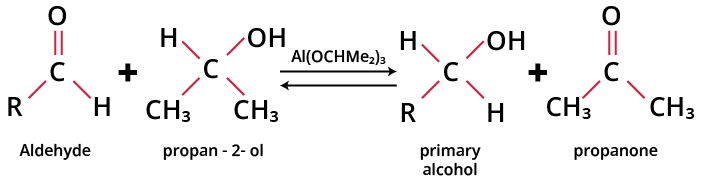
MPV Reduction of Aldehydes
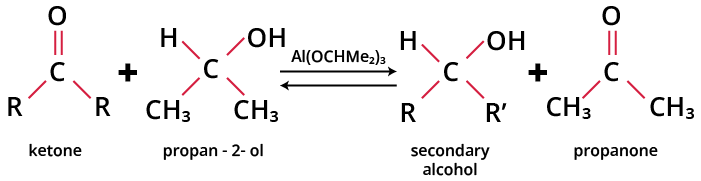
MPV Reduction of Ketones
The equilibrium called the Meerwein-Ponndorf-Verley-Oppenauer equilibrium is shifted to the right by the removal of acetone by distillation.
Conclusion
Aldehydes and ketones can be reduced to hydrocarbons. Clemmenson reduction and Wolff Kishner reduction are the two methods used for reducing aldehydes and ketones to hydrocarbons. In addition, aldehydes can be reduced to primary alcohols and ketones can be reduced to secondary alcohols. Catalytic reduction, metal hydride reduction, and Meerwein-Ponndorf-Verley reduction are the three main methods to accomplish this conversion. In this reduction, lithium aluminium hydride and sodium borohydride are the reducing agents used. There is no change in the end product irrespective of the reducing agent used.
FAQs on Reduction of Aldehydes and Ketones for JEE
1. Lithium aluminum hydride can reduce esters, amides, and carboxylic acids. However, sodium borohydride can’t reduce them. Why?
Usually, carboxylic acids, esters, and amides are less reactive to reduction when compared to aldehydes and ketones. This is because they are better stabilised by the second oxygen atom, which can give one extra lone pair to the polar C=O bond. Moreover, sodium borohydride is a weak reducing agent. Therefore, sodium borohydride can not bring about the reduction of acids, amides, and esters. On the other hand, lithium aluminium hydride is a strong oxidising agent which can reduce these compounds. However, it should be used in a dry ether solution to avoid the reaction with alcohol and water.
2. Which one is more easily reduced, aldehydes or ketones?
Aldehydes are more easily reduced when compared to ketones. This is because ketones are less reactive due to the steric strain induced by the extra bulk alkyl group. This, in turn, can donate electron density to the partial positive charge of the polar C=O bond in ketones. In the case of aldehydes, there is only one alkyl group and the steric strain is low compared to ketones. Therefore, aldehydes are more reactive and require milder reduction agents to be reduced.
























