




What is Decarboxylation?
A decarboxylation reaction is a chemical reaction that involves the removal of a carboxyl group and the release of carbon dioxide (CO2). Decarboxylation is the removal of a carbon atom from a carbon chain by carboxylic acids. In photosynthesis, the separate chemical step when CO2 is added to the material is known as carboxylation. It's an entirely reversible process.
The removal of a carboxyl group (-COOH) from any reactant molecule is referred to as decarboxylation. Decarboxylation is a chemical reaction in which a carboxyl group (-COOH) is removed and carbon dioxide (CO2) is released at the product end. In many circumstances, the release of CO2 makes the reaction almost irreversible.
What is Soda Lime?
Soda lime is a granular mixture of NaOH and CaO chemicals used to remove carbon dioxide from breathing gases in confined breathing conditions such as general anaesthesia, submarines, rebreathers, and recompression chambers to prevent CO2 retention and poisoning. It is used as a harmful gas absorber in gas masks. It is used as a drying agent in laboratories. Soda lime is a highly corrosive poison that, if swallowed, can cause serious damage to the gastrointestinal tract and even death.
Soda lime in granular form
Working Of Soda Lime
The catalytic role of sodium hydroxide in the system is explained by soda lime reaction with CO2 as well as why soda lime is faster in chemical reactivity than calcium hydroxide alone. The surface and porosity of calcium hydroxide grains with a high specific surface area are impregnated with moist NaOH. It reacts significantly faster and hence helps to a faster CO2 removal from the rebreathing circuit. The process produces water, and the moisture produced by respiration acts as a solvent for the reaction. In general, reactions in the aqueous phase are faster than those between a dry gas and a dry solid. In closed-circuit diving rebreathers and anaesthetic systems, soda lime is often used.
$\mathrm{CO}_{2}+\mathrm{CaO} \rightarrow \mathrm{CaCO}_{3}+ \text{heat ( in the presence of water)}$
$\mathrm{CO}_{2}(g) \rightleftharpoons \mathrm{CO}_{2}(a q)\left(\mathrm{CO}_{2} \text { dissolves in water-slowandrate }-\text { determining }\right) \\ $
Soda Lime Decarboxylation
The formula for a carboxylic acid is RCOOH, where R might be hydrogen or a hydrocarbon group like an alkyl group. A benzene ring might also be used to form the hydrocarbon group. A carboxylic acid's sodium salt will have the formula RCOONa. The -COOH or -COONa group is eliminated and replaced with a hydrogen atom during decarboxylation. The sodium hydroxide solution is added to solid calcium oxide to make soda lime (quicklime). Sodium hydroxide, calcium oxide, and calcium hydroxide are the main components. It occurs in the form of white granules. It is almost always written as sodium hydroxide in equations.
Solid sodium hydroxide is a difficult material to work with. If you leave solid sodium hydroxide exposed to the air, it will absorb water from the atmosphere, resulting in puddles of highly concentrated (and corrosive) sodium hydroxide solution. Soda lime has a lower absorption rate than regular lime.
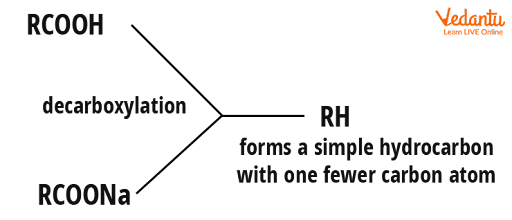
Decarboxylation Of Carboxylic Acid To Hydrocarbon
Let’s now see, what happens when carboxylic acid is treated with soda lime
A carboxylic acid's solid sodium salt is combined with solid soda lime, and the mixture is heated. When sodium ethanoate is heated with soda lime, for example, methane gas is produced. Three steps are involved in the reaction's mechanism. The first involves removing the H+ ion from the carboxylic acid and replacing it with Na. This procedure resulted in the release of a sodium salt of carboxylic acid and a water molecule.
A negative charge from oxygen is transported between the carbon-oxygen bond in the second phase. As a result, the alkyl group(-R) and carbon bond splits. In this stage, CO2 is released.
The third phase is carried out with the assistance of H2O created as a result of the first step It splits to release a proton ion, which reacts with an alkyl group to form an alkane.

Sodium salt of carboxylic acid with soda lime
Some examples of soda lime decarboxylation
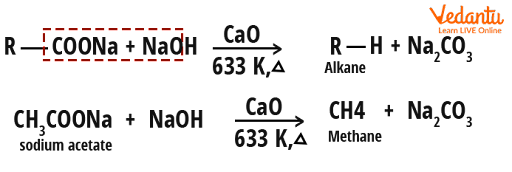
Examples of soda lime decarboxylation
Rate Of Decarboxylation Reaction
Decarboxylation occurs more quickly in substances with high unsaturation levels. Double bond(s), triple bond(s), and/or ring(s) are all present in unsaturated molecules (s). Another way to look at it is that a saturated molecule has the most hydrogen atoms. Determine which chemicals are highly unsaturated.
Another aspect is the presence of electron withdrawing groups in carboxylic acid. The higher the rate the faster will be decarboxylation.
Summary
Soda lime is a granular mixture of NaOH and CaO chemicals used to remove carbon dioxide from breathing gases. A carboxylic acid's solid sodium salt is combined with solid soda lime, and the mixture is heated. This results in the decarboxylation of carboxylic acid.Decarboxylation occurs more quickly in substances with high unsaturation levels. Double bond(s), triple bond(s), and/or ring(s) are all present in unsaturated molecules. Another way to look at it is that a saturated molecule has the most hydrogen atoms. Determine which chemicals are highly unsaturated.
FAQs on Soda Lime Decarboxylation for JEE
1. Define Decarboxylation Enzymes?
The removal of carbon dioxide from organic acids, known as decarboxylation, is a crucial event in biology. Decarboxylase enzymes play an important role in glucose metabolism and amino acid conversion in both aerobic and anaerobic environments. Decarboxylases or carboxy-lyases are enzymes that aid in the decarboxylation of a variety of organic acids. They have the ability to add and remove carboxyl groups from organic compounds. They are frequently given names based on the substrates that they catalyse. Ornithine decarboxylase, RuBisCO, Pyruvate decarboxylase, Histidine decarboxylase, and others are examples of these enzymes.
2. What are decarboxylation tests?
These are biochemical tests that involve enzyme decarboxylase synthesis. As a result, they're sometimes called decarboxylase tests.The test is used to distinguish decarboxylase-producing Enterobacteriaceae bacteria from other gram-negative bacteria.The development of decarboxylase enzymes, such as arginine decarboxylase, ornithine decarboxylase, and lysine decarboxylase, are used to identify organisms that can decarboxylate amino acids.The ability to manufacture these enzymes is then used to further differentiate the members.Decarboxylation is detected by adding substrates like arginine, ornithine, and lysine to the medium.
























