




How Does CFSE Affect the Stability of Coordination Complexes?
Understanding the stability of complexes and CFSE (Crystal Field Stabilisation Energy) is essential for mastering transition metal chemistry in JEE Main. These concepts guide why certain coordination compounds are more stable than others and how electron arrangement, ligands, and geometry influence the energy of a metal complex. Grasping the calculation, application, and factors affecting CFSE will help you answer typical JEE MCQs swiftly and accurately.
What is Stability of Complexes and CFSE?
The stability of complexes refers to their resistance to breaking down or exchanging ligands. It is quantified as either thermodynamic stability (how energetically favourable a complex is) or kinetic stability (how quickly ligand exchange occurs). CFSE is the additional stabilisation a central metal ion receives when its d-orbitals split, due to the electric field from surrounding ligands.
Factors Affecting Stability of Complexes
Several factors determine the stability of coordination complexes for JEE:
- Size of central metal ion: Smaller ions attract ligands more effectively, increasing stability.
- Charge on metal ion: Higher charge means stronger interaction and higher complex stability.
- Nature of ligands: Strong-field ligands (like CN-, NH3) give more stable complexes than weak-field ligands (like F-, Cl-).
- Steric effects: Bulky ligands reduce stability due to repulsion.
- Chelate and macrocyclic effect: Multidentate and macrocyclic ligands form more stable complexes.
For example, [Co(en)3]3+ is more stable than [Co(NH3)6]3+ because of the chelation effect.
Crystal Field Stabilisation Energy (CFSE)
Crystal Field Stabilisation Energy is the difference in energy between the d-electrons in the presence of a ligand field and in a spherical field. When ligands approach a transition metal ion, the five d-orbitals split into two sets at different energies. For octahedral geometry, the dz2 and dx2-y2 orbitals (eg) are raised in energy, while dxy, dyz, and dxz (t2g) are stabilised. The resulting energy stabilisation is called CFSE and directly relates to stability.
Crystal field splitting in octahedral complexes leads to an arrangement where ligands along the axes cause splitting of d-orbitals. The quantum of splitting (Δo) depends on ligands and metal ion properties.
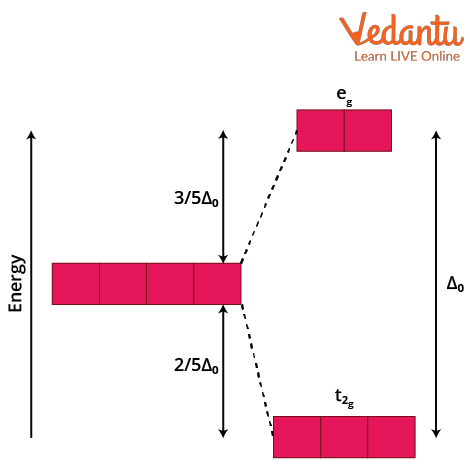
Higher CFSE usually means a more stable complex, as the metal-ligand combination leads to lower overall energy for the arrangement.
CFSE Calculation and Formula Guide
For JEE Main, you should know how to calculate CFSE for both octahedral and tetrahedral complexes. The formulas for CFSE (in terms of Δ) are:
- Octahedral: CFSE = [-0.4 × (number of t2g electrons) + 0.6 × (number of eg electrons)]×Δo
- Tetrahedral: CFSE = [-0.6 × (number of t2 electrons) + 0.4 × (number of e electrons)]×Δt
- For most tetrahedral complexes, Δt = 4/9 × Δo
Electron pairing energy (P) must also be added where electrons pair in the same orbital. High-spin (weak-field) and low-spin (strong-field) configurations can give different CFSE values.
Stepwise CFSE Calculation Example (Octahedral d6)
Suppose you have [Fe(CN)6]4- (Fe2+, d6, strong field, low spin):
- Lowest energy filling leads to t2g6eg0 configuration.
- CFSE = [-0.4 × 6 + 0.6 × 0]Δo = -2.4Δo (maximum stabilisation for d6 low spin)
- Pairing energy is also added if asked.
For high spin [Fe(H2O)6]2+, electrons are distributed t2g4eg2, so CFSE = [-0.4×4+0.6×2]=-0.4Δo.
Comparison: Octahedral vs Tetrahedral vs Square Planar Complexes
| Geometry | d-Orbital Splitting | CFSE Trend |
|---|---|---|
| Octahedral | t2g--eg | CFSE higher |
| Tetrahedral | e--t2 | CFSE lower (~44% of oct) |
| Square planar | Greatest splitting; often low-spin, for d8 | Can be very stable |
Octahedral complexes are most common, and usually more stable than tetrahedral due to greater crystal field splitting. Square planar geometry (like [Ni(CN)4]2-) is favoured by low spin d8 ions.
How Ligands Influence CFSE and Complex Stability
Ligand strength (spectrochemical series) is a key factor in stability and CFSE. Strong-field ligands (CN-, en, NO2-) increase crystal field splitting and thus stabilisation. Weak-field ligands (F-, Cl-, H2O) give smaller splitting and less CFSE.
- Different ligands cause the same metal to have varied stability in complexes.
- Chelating ligands increase stability due to the chelate and macrocyclic effect.
- Spectrochemical series arranges ligands according to increasing field strength.
For exam problems, relate the 'strength' of the ligand (from the series) to the likely spin state and CFSE outcome.
Quick Notes and Revision Table
| Key Aspect | Statement |
|---|---|
| Stability Order | Macrocyclic > Chelate > Monodentate |
| CFSE Formula (oct) | -0.4nt2g + 0.6neg |
| Oct vs Tet | Δt = (4/9)Δo |
| Spin state | Strong field ligands: Low spin (max CFSE) |
| Main JEE trap | Mixing pairing energy with stabilisation energy |
Revise the formulae and key trends above before attempting MCQs or numericals. Practice with solved questions to gain speed and confidence.
Handy Problem-Solving Strategies for JEE
- Write electronic configuration correctly for the metal’s oxidation state.
- Identify ligand strength using the spectrochemical series for likely spin state.
- Calculate CFSE, considering pairing energy (P) if mentioned.
- For stability comparison, use magnitude of CFSE and consider chelate effect.
- Memorise maximum CFSE values for d3, d6 (low spin).
- Practice papers boost speed in recognising exam patterns.
Why Stability of Complexes and CFSE Matters in JEE Main
A strong grasp of stability of complexes and CFSE empowers you to solve JEE level coordination chemistry questions rapidly. With the stepwise calculation method, logic for comparing stability, and memory of CFSE formulae, you can tackle both direct and conceptual MCQs confidently. Experienced educators at Vedantu recommend regular revision of formulae, solved examples, and quick practice with concept maps and tables, ensuring this topic turns into a reliable scoring area.
- Coordination compounds overview
- States of matter – relevant linkage
- Co-ordinate bonding explained
- Coordination compounds mock tests
- Revision notes for coordination chemistry
- Important questions on complexes
- Ligands and types for JEE
- Extra MCQs: coordination compounds
- Test your complex stability knowledge
- Substance states – chemistry link
- Full JEE chemistry syllabus
Vedantu supports your JEE Main journey by presenting clear, syllabus-driven explanations and up-to-date solved practice items, making tricky topics like the stability of complexes and CFSE straight-forward to master.
FAQs on Stability of Complexes and Crystal Field Stabilization Energy (CFSE)
1. What is the relationship between stability and CFSE?
Stability of a coordination complex increases as its Crystal Field Stabilization Energy (CFSE) becomes more negative. This means that the greater the CFSE, the more stable the complex. Important points include:
- Higher (more negative) CFSE lowers the overall energy of a metal complex.
- Ligand type and geometry (octahedral, tetrahedral) affect CFSE values.
- Complexes with maximum CFSE resist dissociation, showing higher stability in solution.
2. How do you calculate CFSE for an octahedral complex?
To calculate the Crystal Field Stabilization Energy (CFSE) for an octahedral complex, follow these steps:
- Determine the distribution of d-electrons in the t2g and eg orbitals based on electron count and spin state.
- Use the formula: CFSE = [(-0.4 × number of t2g electrons) + (0.6 × number of eg electrons)] Δo.
- Subtract pairing energy if electron pairing is involved.
- Assign Δo (octahedral crystal field splitting energy) using ligand types (from the spectrochemical series).
- Add values for total CFSE.
3. What factors affect the stability of a coordination complex?
Several factors influence the stability of a coordination complex:
- Ligand strength: Strong field ligands increase CFSE and thus stability.
- Charge on metal ion: Higher charge favors higher stability.
- Nature of ligand: Chelating ligands increase complex stability via the chelate effect.
- Geometry of the complex: Octahedral, tetrahedral, and square planar differ in stability.
- Size of metal ion: Smaller size generally increases stability.
4. What is the effect of ligand strength on CFSE?
Stronger ligands cause larger crystal field splitting, leading to higher CFSE values and increased complex stability.
- Spectrochemical series ranks ligands by their field strength.
- Strong field ligands (like CNˉ, CO) induce higher splitting and favor low spin complexes.
- Weak field ligands (like Clˉ, Fˉ) result in lower splitting and high spin complexes.
5. What is Crystal Field Stabilization Energy (CFSE)?
Crystal Field Stabilization Energy (CFSE) is the energy difference that results from the arrangement of ligands around a metal ion, lowering the overall energy of the complex.
- CFSE quantifies the relative stability of coordination compounds.
- It is calculated based on the splitting of d-orbitals into t2g and eg levels in different geometries (octahedral/tetrahedral).
- A more negative CFSE value indicates a more stable complex.
6. How does geometry (octahedral vs tetrahedral) impact the value of CFSE for the same metal-ligand combination?
Octahedral complexes have larger CFSE values compared to tetrahedral complexes for the same metal and ligands.
- CFSE (octahedral) = -0.4 × t2g + 0.6 × eg electrons × Δo
- CFSE (tetrahedral) = -0.6 × t2 + 0.4 × e electrons × &Delta>t (but &Delta>t < &Delta>o)
- Octahedral geometry leads to stronger ligand field splitting and greater stabilization.
7. Why is CFSE negative for most stable complexes?
A negative CFSE signifies energy release upon complex formation, making the complex more stable.
- The splitting of d-orbitals in a crystal field lets some electrons occupy lower-energy orbitals, releasing energy.
- Lower energy means greater stability for the metal-ligand complex.
8. Is CFSE alone sufficient to predict all aspects of complex stability?
CFSE is important, but not the sole factor influencing complex stability.
- Other contributions include pairing energy, ligand-metal bond strength, chelate effect, and entropy changes.
- CFSE gives good predictions for trends, but full stability requires considering all energetic and structural implications.
9. What increases or decreases the stability of coordination complexes?
The stability of coordination complexes is increased by:
- High charge density on metal ions
- Strong field ligands (CN−, CO)
- Chelating ligands (e.g., EDTA)
- Appropriate geometry for maximum CFSE (octahedral for most)
- Weak field ligands (F−, Cl−)
- Large, low charge metal ions
- Ligand-ligand repulsion or steric hindrance
10. How does pairing energy influence the final CFSE and stability?
Pairing energy (P) opposes the stabilization gained from CFSE.
- If pairing energy is high, electrons prefer occupying higher orbitals, reducing CFSE benefits.
- When CFSE > pairing energy, low-spin complexes form and are more stable.
- If CFSE < pairing energy, high-spin complexes and less stabilization result.























