
Consider all possible isomeric ketones, including stereoisomers, of MW = 100. All these isomers are independently reacted with $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$ (NOTE: stereoisomers are also reacted separately). The total number of ketones that give a racemic product(s) is/are:
Answer
232.8k+ views
Hint: The general molecular formula of ketone containing ’n’ carbon atom is given as, $\text{ }{{\text{C}}_{\text{n}}}{{\text{H}}_{\text{2n}}}\text{O }$ .The molecular weight is equal to the sum of the product of the atomic mass of element and number of an atom in the compound. The isomers are the structure having the same molecular formula but the difference in the position of the functional group. This is drawn in such a way that they fully fill the molecular formula of the compound. The $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$ is a hydride rich complex.it reduces the carbonyl group to the alcohol and generates stereocenters.
Complete step by step solution:
We will solve this question in parts.
A) Determination of the ketone compound:
We have given a ketone compound with a molecular weight equal to 100. The general molecular formula of ketone containing ’n’ carbon atom is given as,$\text{ }{{\text{C}}_{\text{n}}}{{\text{H}}_{\text{2n}}}\text{O }$ .
Let's find out the value of the number of carbon atom ‘n’.Here, the molecular weight would be equal to,
$\begin{align}
& \text{ n}\times \text{ 12 + 2n }\times \text{ 1 + 16 }=100 \\
& \Rightarrow \text{n = }\frac{100-16}{14}=\frac{84}{14}\text{ = 6 } \\
\end{align}$
Thus the ketone compound contains 6 carbon atoms and the molecular formula is $\text{ }{{\text{C}}_{6}}{{\text{H}}_{12}}\text{O }$.
B) Determination of all possible isomer of$\text{ }{{\text{C}}_{6}}{{\text{H}}_{12}}\text{O }$:
Here, we will found out all possible isomer of the $\text{ }{{\text{C}}_{6}}{{\text{H}}_{12}}\text{O }$ molecular formula. Isomer is a molecule that has the same molecular formula but the difference in the attachment of atoms. Thus to find the possible isomers arranged the six-carbon, twelve hydrogens, and one carbonyl oxygen in various forms. These are as tabulated as follows,
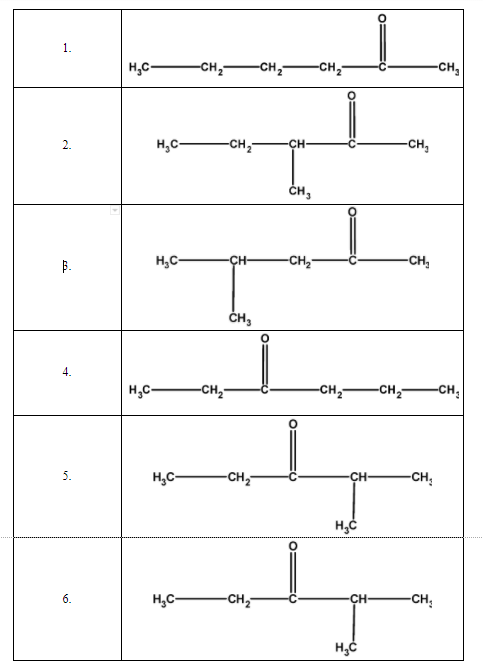
There are a total of 6 structural isomers for the $\text{ }{{\text{C}}_{6}}{{\text{H}}_{12}}\text{O }$formula.
C) Reaction with $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$ :
Aldehyde or ketone on catalytic reduction in presence of $\text{ Ni }$ , $\text{ Pt }$ , $\text{ Pd }$ or chemically by complex metal hydrides such as sodium borohydride $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$gives alcohol. Ketones on the reduction with $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$giving secondary alcohols.
The general reaction of the carbonyl $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$is as follows,
$\text{ }-\underset{|}{\overset{|}{\mathop{\text{C}}}}\,\text{=O }\xrightarrow{\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }}\text{ H}-\underset{|}{\overset{|}{\mathop{\text{C}}}}\,-\text{OH }$
Here, the above-obtained isomer reacts with the $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$. The reaction results in the reduction of the carbonyl group into the alcohol. The reaction may generate a chiral or stereogenic centre at the carbon atom. If a compound has a chiral carbon atom, then it exhibits optical activity. Let's consider an example of one of the isomers from the given table,
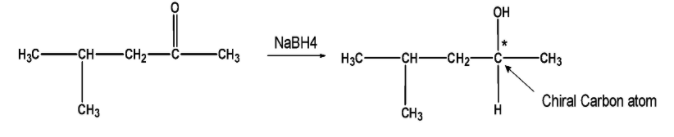
Likewise, out of six isomer five isomers on reduction forms the chiral carbon atom, this further leads to the R and S configuration at the stereogenic center.There is an equal possibility for the compound to exist in two enantiomeric forms (R and S).
The following isomer on the reduction gives secondary alcohol. On a closer look, the compound has two stereocenters adjacent to each other. Thus it does not form the racemic mixture.
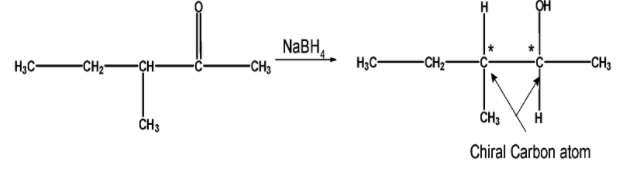
Thus a total of five isomers form the racemic mixture on reduction.
Hence, the answer is 5.
Note: Note that, if a ketone already has a chiral centre and undergoes the reduction it will form another chiral centre in the compound. These chiral centres are adjacent to each other, compounds from the diastereomers. These stereoisomers do not have mirror relationships like enantiomers and therefore they do not form a racemic mixture.
Complete step by step solution:
We will solve this question in parts.
A) Determination of the ketone compound:
We have given a ketone compound with a molecular weight equal to 100. The general molecular formula of ketone containing ’n’ carbon atom is given as,$\text{ }{{\text{C}}_{\text{n}}}{{\text{H}}_{\text{2n}}}\text{O }$ .
Let's find out the value of the number of carbon atom ‘n’.Here, the molecular weight would be equal to,
$\begin{align}
& \text{ n}\times \text{ 12 + 2n }\times \text{ 1 + 16 }=100 \\
& \Rightarrow \text{n = }\frac{100-16}{14}=\frac{84}{14}\text{ = 6 } \\
\end{align}$
Thus the ketone compound contains 6 carbon atoms and the molecular formula is $\text{ }{{\text{C}}_{6}}{{\text{H}}_{12}}\text{O }$.
B) Determination of all possible isomer of$\text{ }{{\text{C}}_{6}}{{\text{H}}_{12}}\text{O }$:
Here, we will found out all possible isomer of the $\text{ }{{\text{C}}_{6}}{{\text{H}}_{12}}\text{O }$ molecular formula. Isomer is a molecule that has the same molecular formula but the difference in the attachment of atoms. Thus to find the possible isomers arranged the six-carbon, twelve hydrogens, and one carbonyl oxygen in various forms. These are as tabulated as follows,

There are a total of 6 structural isomers for the $\text{ }{{\text{C}}_{6}}{{\text{H}}_{12}}\text{O }$formula.
C) Reaction with $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$ :
Aldehyde or ketone on catalytic reduction in presence of $\text{ Ni }$ , $\text{ Pt }$ , $\text{ Pd }$ or chemically by complex metal hydrides such as sodium borohydride $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$gives alcohol. Ketones on the reduction with $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$giving secondary alcohols.
The general reaction of the carbonyl $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$is as follows,
$\text{ }-\underset{|}{\overset{|}{\mathop{\text{C}}}}\,\text{=O }\xrightarrow{\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }}\text{ H}-\underset{|}{\overset{|}{\mathop{\text{C}}}}\,-\text{OH }$
Here, the above-obtained isomer reacts with the $\text{ NaB}{{\text{H}}_{\text{4}}}\text{ }$. The reaction results in the reduction of the carbonyl group into the alcohol. The reaction may generate a chiral or stereogenic centre at the carbon atom. If a compound has a chiral carbon atom, then it exhibits optical activity. Let's consider an example of one of the isomers from the given table,

Likewise, out of six isomer five isomers on reduction forms the chiral carbon atom, this further leads to the R and S configuration at the stereogenic center.There is an equal possibility for the compound to exist in two enantiomeric forms (R and S).
The following isomer on the reduction gives secondary alcohol. On a closer look, the compound has two stereocenters adjacent to each other. Thus it does not form the racemic mixture.

Thus a total of five isomers form the racemic mixture on reduction.
Hence, the answer is 5.
Note: Note that, if a ketone already has a chiral centre and undergoes the reduction it will form another chiral centre in the compound. These chiral centres are adjacent to each other, compounds from the diastereomers. These stereoisomers do not have mirror relationships like enantiomers and therefore they do not form a racemic mixture.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




