
Draw the structure of a butanone molecule.
Answer
232.8k+ views
Hint: Attempt This question by breaking the given molecule to decipher its meaning. For example – methane can be broken to ‘meth-‘+ ‘-ane’, which means one carbon and alkane respectively.
Complete step by step answer:
Butanone, as the name suggests, is made up of “but- “+ “-ane” + “-one”. Therefore, we can see the following points from it –
‘but- ‘stands for four carbons.
‘-ane’ suggests that it is an alkane
‘-one’ suggests that it is a ketone.
Therefore, we can draw the structure as –
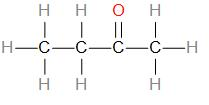
As we can see, the given compound is a ketone, therefore, double bonded oxygen can only be placed at the second carbon. The compound can also be called Methyl ethyl ketone.
We can write its compressed formula as – \[C{{H}_{3}}C{{H}_{2}}COC{{H}_{3}}\].
Butanone is an organic compound, which appears as a colorless liquid with a sharp and sweet odor. This compound is produced industrially on a large scale, but it occurs scarcely in nature. It is partially soluble in water and is commonly used as an industrial solvent. Also, it is an isomer of tetrahydrofuran.
Additional information: Root words - meth, eth, prop, but, pent are words which define the number of carbons in the compound.
Note: Butanone can be produced by oxidation of 2-butanol. The dehydrogenation of 2-butanol is catalyzed by copper, zinc, or bronze. Liquid-phase oxidation of heavy naphtha and the Fischer-Tropsch reaction, both produce mixed oxygenate streams, from which 2-butanone is extracted by fractionation.
Complete step by step answer:
Butanone, as the name suggests, is made up of “but- “+ “-ane” + “-one”. Therefore, we can see the following points from it –
‘but- ‘stands for four carbons.
‘-ane’ suggests that it is an alkane
‘-one’ suggests that it is a ketone.
Therefore, we can draw the structure as –

As we can see, the given compound is a ketone, therefore, double bonded oxygen can only be placed at the second carbon. The compound can also be called Methyl ethyl ketone.
We can write its compressed formula as – \[C{{H}_{3}}C{{H}_{2}}COC{{H}_{3}}\].
Butanone is an organic compound, which appears as a colorless liquid with a sharp and sweet odor. This compound is produced industrially on a large scale, but it occurs scarcely in nature. It is partially soluble in water and is commonly used as an industrial solvent. Also, it is an isomer of tetrahydrofuran.
Additional information: Root words - meth, eth, prop, but, pent are words which define the number of carbons in the compound.
Note: Butanone can be produced by oxidation of 2-butanol. The dehydrogenation of 2-butanol is catalyzed by copper, zinc, or bronze. Liquid-phase oxidation of heavy naphtha and the Fischer-Tropsch reaction, both produce mixed oxygenate streams, from which 2-butanone is extracted by fractionation.
Recently Updated Pages
Area of an Octagon Formula Explained Simply

Absolute Pressure Formula Explained: Key Equation & Examples

Central Angle of a Circle Formula Explained Quickly

Difference Between Vapor and Gas: JEE Main 2026

Difference Between Atom and Molecule: JEE Main 2026

Carbon Dioxide Formula - Definition, Uses and FAQs

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Jan 21 Shift 1 Question Papers with Solutions & Answer Keys – Detailed Day 1 Analysis

JEE Main Response Sheet 2026 Released – Key Dates and Official Updates by NTA

JEE Main 2026 Answer Key OUT – Download Session 1 PDF, Response Sheet & Challenge Link

JEE Main Marks vs Percentile 2026: Calculate Percentile and Rank Using Marks

JEE Main 2026 Jan 22 Shift 1 Today Paper Live Analysis With Detailed Solutions

Other Pages
Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages

One Day International Cricket

Valentine Week 2026: Complete List of Valentine Week Days & Meaning of Each Day

List of Highest T20 Scores in International Cricket

Makar Sankranti Wishes: Happy Makar Sankranti Wishes in Marathi, Hindi, Kannada, and English

What is the Full Form of UGC? Detailed Guide for Students




