
Equivalent conductance for strong electrolyte on dilution _____.
Answer
232.8k+ views
Hint: The conducting power of the ions present in the solution which is formed by dissolving , electrolyte of 1 gram equivalent in a particular solvent is called equivalent conductance.
The equivalent conductance is denoted by \[{\Lambda _e}\].
Complete step by step solution:
Dilution is defined as a process where the solute concentration is decreased with the addition of solvent.
The variations in the value of equivalent conductance depend on the type of electrolyte.
Strong electrolytes dissociate completely into its substituent ions when dissolved in solvent forming a solution. On increasing the dilution (decreasing the concentration of solute), there is the decline in the attractive force present between the cation and anion which further affect there mobility towards each other. This is known as ionic interference. Due to the increase in dilution, the equivalent conductance increases and at infinite dilution, the value of equivalent conductance is highest and it is termed as equivalent conductance at infinite dilution.
Therefore, the equivalent conductance for strong electrolyte on dilution increases.
The effect of electrolyte concentration on the equivalent conductance is observed by the graph plotted between the equivalent conductance value and square root of concentration.
The graph for strong electrolyte is shown below.
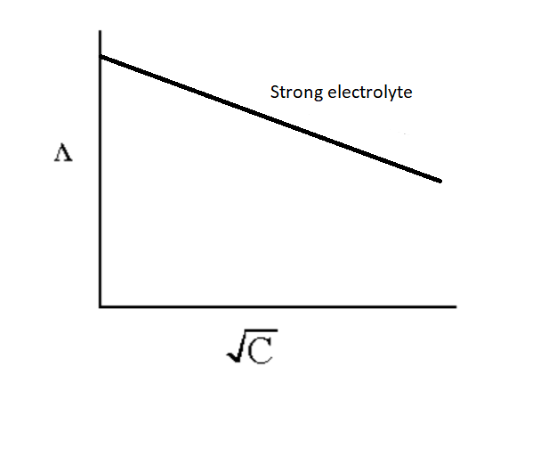
Image: Effect of dilution in the value of equivalent conductance
Note: For strong electrolyte, the number of ions remains the same in the solution at all dilutions, the only change observed is the mobility of ions due to the effect of interionic attraction but in the case of weak electrolyte, the increase in the value of equivalent conductance is due to the increase in the number of ions as weak electrolyte possess a low concentration of ions, so the interionic interaction is negligible.
The equivalent conductance is denoted by \[{\Lambda _e}\].
Complete step by step solution:
Dilution is defined as a process where the solute concentration is decreased with the addition of solvent.
The variations in the value of equivalent conductance depend on the type of electrolyte.
Strong electrolytes dissociate completely into its substituent ions when dissolved in solvent forming a solution. On increasing the dilution (decreasing the concentration of solute), there is the decline in the attractive force present between the cation and anion which further affect there mobility towards each other. This is known as ionic interference. Due to the increase in dilution, the equivalent conductance increases and at infinite dilution, the value of equivalent conductance is highest and it is termed as equivalent conductance at infinite dilution.
Therefore, the equivalent conductance for strong electrolyte on dilution increases.
The effect of electrolyte concentration on the equivalent conductance is observed by the graph plotted between the equivalent conductance value and square root of concentration.
The graph for strong electrolyte is shown below.

Image: Effect of dilution in the value of equivalent conductance
Note: For strong electrolyte, the number of ions remains the same in the solution at all dilutions, the only change observed is the mobility of ions due to the effect of interionic attraction but in the case of weak electrolyte, the increase in the value of equivalent conductance is due to the increase in the number of ions as weak electrolyte possess a low concentration of ions, so the interionic interaction is negligible.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




