
Give the structures and IUPAC names of monohydric phenols of molecular formula, ${C_7}{H_8}O.$
Answer
233.1k+ views
Hint: Phenols are aromatic alcohols. Monohydric phenol has one hydroxyl group attached to a benzene ring.
Complete step by step answer:
Molecular formula of the compound is ${C_7}{H_8}O.$ We have to find monohydric phenols. Phenols are aromatic alcohols in which one hydroxyl group is attached to a benzene ring.
Benzene ring has 6-carbon atoms in a ring, but total 7-carbon atoms are given in molecular formula.
Therefore, each phenol is methyl substituted phenol. They are: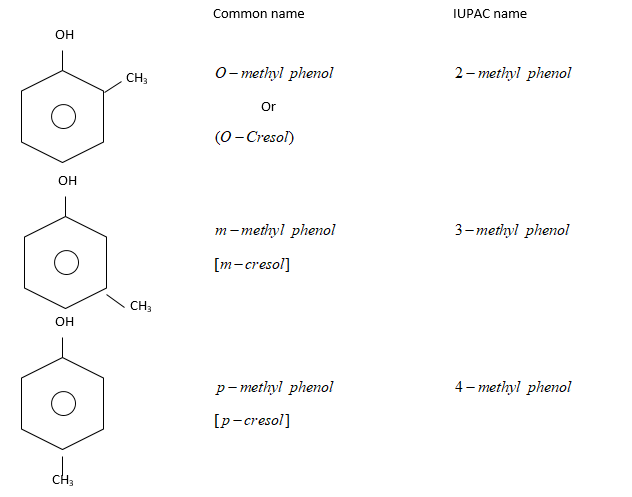 Common name of monohydric phenol is cresol.
Common name of monohydric phenol is cresol.
When two hydrogen atoms of benzene are replaced by two monovalent atom or group of atoms, then resulting product have three different forms:
(i) Ortho (or 1,2): Substituents present on adjacent carbon atom. i.e., $ - C{H_3}$and $ - OH$group are present at adjacent C-atom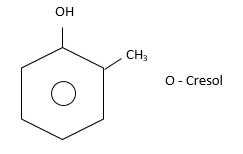
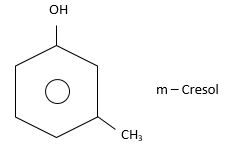
(ii) Meta (or 1,3): Substituents are present on alternate C-atoms. i.e., $ - C{H_3}$ and $ - OH$are present on alternate C-atoms.
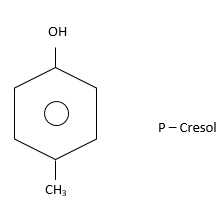
(iii) Para (or 1,4): Two substituents i.e. $ - C{H_3}$ and $ - OH$are present on diagonally situated $C - atoms.$
Ortho, meta and para are generally represented as $O - ,m - $ and $p - $respectively.
Note: The formation of different structures by using one molecular formula is called isomerism. In the given compound three isomers are formed in which position of $ - C{H_3}$group is different with respect to $ - OH$ group on benzene ring.

Complete step by step answer:
Molecular formula of the compound is ${C_7}{H_8}O.$ We have to find monohydric phenols. Phenols are aromatic alcohols in which one hydroxyl group is attached to a benzene ring.
Benzene ring has 6-carbon atoms in a ring, but total 7-carbon atoms are given in molecular formula.
Therefore, each phenol is methyl substituted phenol. They are:
 Common name of monohydric phenol is cresol.
Common name of monohydric phenol is cresol.When two hydrogen atoms of benzene are replaced by two monovalent atom or group of atoms, then resulting product have three different forms:

(i) Ortho (or 1,2): Substituents present on adjacent carbon atom. i.e., $ - C{H_3}$and $ - OH$group are present at adjacent C-atom

(ii) Meta (or 1,3): Substituents are present on alternate C-atoms. i.e., $ - C{H_3}$ and $ - OH$are present on alternate C-atoms.

(iii) Para (or 1,4): Two substituents i.e. $ - C{H_3}$ and $ - OH$are present on diagonally situated $C - atoms.$

Ortho, meta and para are generally represented as $O - ,m - $ and $p - $respectively.
Note: The formation of different structures by using one molecular formula is called isomerism. In the given compound three isomers are formed in which position of $ - C{H_3}$group is different with respect to $ - OH$ group on benzene ring.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




