
If work done by an ideal diatomic gas in a process from $\mathrm{A}$ to $\mathrm{B}$ as shown in $\mathrm{P}-\mathrm{V}$ graph is $20 \mathrm{J}$ :
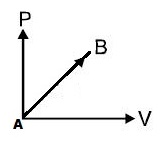
Then increase in internal energy of the gas is:
(A) $40 \mathrm{J}$
(B) $60 \mathrm{J}$
(C) $100 \mathrm{J}$
(D) $120 \mathrm{J}$
Answer
232.8k+ views
Hint: We know that the internal energy is an extensive property: it depends on the size of the system, or on the amount of substance it contains. At any temperature greater than absolute zero, microscopic potential energy and kinetic energy are constantly converted into one another, but the sum remains constant in an isolated system. The internal energy of a system can be understood by examining the simplest possible system: an ideal gas. Because the particles in an ideal gas do not interact, this system has no potential energy. The internal energy of an ideal gas is therefore the sum of the kinetic energies of the particles in the gas.
Complete step by step answer
We know that the internal energy of an ideal gas is therefore the sum of the kinetic energies of the particles in the gas. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles. All objects contain internal energy. ... When an object is heated, its particles move more vigorously and its internal energy increases. Unless the object changes state (e.g. melts or boils), its temperature will increase.
$\mathrm{W}=$ Area $=\dfrac{1}{2} \mathrm{P} \mathrm{V}=\dfrac{1}{2} \mathrm{nR} \Delta \mathrm{T}=20 \mathrm{J}$
Now the change in internal energy is given as $\dfrac{\mathrm{f}}{2} \mathrm{nR} \Delta \mathrm{T}$ (here $\mathrm{f}$ is the number of degree of freedom)
For a diatomic gas $f=5 .$ Thus we get $\Delta \mathrm{U}=\dfrac{5}{2} \mathrm{nR} \Delta \mathrm{T}=100 \mathrm{J} .=\mathrm{x}$
The number 100 is a perfect square and it has 9 factors including 1, 2, 4, 5, 10, 20, 25, 50 and 100
So the correct answers are option A and B.
Note: We know that the internal energy of a system is identified with the random, disordered motion of molecules; the total (internal) energy in a system includes potential and kinetic energy. The internal energy is the total amount of kinetic energy and potential energy of all the particles in the system. Whether the energy breaks bonds, increases the speed of the particles to stretch bonds, or just increases the speed of the particles depends on the temperature and state of the material. It depends on the quantity of a substance; hence it is extensive property. Change in it represents the heat evolved or absorbed in a reaction at constant temperature and constant volume.
Complete step by step answer
We know that the internal energy of an ideal gas is therefore the sum of the kinetic energies of the particles in the gas. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles. All objects contain internal energy. ... When an object is heated, its particles move more vigorously and its internal energy increases. Unless the object changes state (e.g. melts or boils), its temperature will increase.
$\mathrm{W}=$ Area $=\dfrac{1}{2} \mathrm{P} \mathrm{V}=\dfrac{1}{2} \mathrm{nR} \Delta \mathrm{T}=20 \mathrm{J}$
Now the change in internal energy is given as $\dfrac{\mathrm{f}}{2} \mathrm{nR} \Delta \mathrm{T}$ (here $\mathrm{f}$ is the number of degree of freedom)
For a diatomic gas $f=5 .$ Thus we get $\Delta \mathrm{U}=\dfrac{5}{2} \mathrm{nR} \Delta \mathrm{T}=100 \mathrm{J} .=\mathrm{x}$
The number 100 is a perfect square and it has 9 factors including 1, 2, 4, 5, 10, 20, 25, 50 and 100
So the correct answers are option A and B.
Note: We know that the internal energy of a system is identified with the random, disordered motion of molecules; the total (internal) energy in a system includes potential and kinetic energy. The internal energy is the total amount of kinetic energy and potential energy of all the particles in the system. Whether the energy breaks bonds, increases the speed of the particles to stretch bonds, or just increases the speed of the particles depends on the temperature and state of the material. It depends on the quantity of a substance; hence it is extensive property. Change in it represents the heat evolved or absorbed in a reaction at constant temperature and constant volume.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




