
In electro-refining of copper, some gold is deposited as:
(A) Cathode mud
(B) Electrolyte
(C) Anode mud
(D) None of these
Answer
232.8k+ views
Hint: The Refining itself means to remove impurities or undesirable substances from a substance. So, electro-refining is a process to remove impurities using electricity. Basically, it's a process of refining in metals using electrolysis.
Complete step by step answer:
Here we will take an example of electrolytic copper refining to understand the process more clearly. Copper is typically mined from its coal referred to as copper. It is about 98 to 99 percent pure. However, the electro-refining process can easily make it 99.95% pure which makes it a good product to be used in electrical components.
A block of impure copper is taken as an anode or positive electrode. Copper sulphate which is acidified with vitriol is employed as a graphite-coated electrolyte alongside pure copper tubes, as a cathode or negative electrode. In this phase of electrolysis copper sulphate divides into a positive ion of copper ($C{{u}^{2+}}$) and a negative ion of sulphate ($S{{O}_{4}}^{-}$). The positive copper ion ($C{{u}^{2+}}$) or cations travel towards the negative electrode made from pure copper where it absorbs the electrons from the cathode. Cu atoms are deposited on the cathode’s graphite layer.
The cathode is coated with graphite in the process of electrolytic metal processing or merely electro grinding so that the concentrated material can be easily removed. This is one of the most growing electrolysis procedures.
Electrolytic Refining of Copper
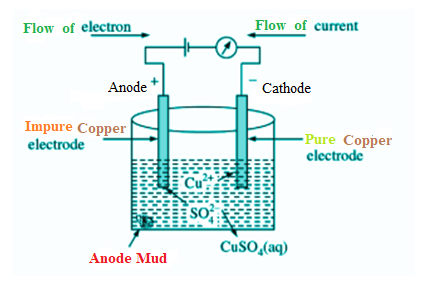
However, anode’s metallic impurities are also mixed with $S{{O}_{4}}$, forming metallic sulphate in the electrolyte solution and dissolving. In this above process, some impurities get dissolved in the solution while some deposits as anode mud below the anode electrode. Impurities which dissolve within the solution are Fe, Ni, Zn while impurities like Au, Ag and Pt deposits as anode mud below the anode electrode.
So, we can say that gold (Au) is deposited as Anode mud below the anode electrode.
Therefore, among the options if we check then option (C)Anode mud is the correct answer of the question.
Note:
- ${{H}^{+}}$from the electrolyte isn't reduced to ${{H}_{2}}$ (g) at the cathode.
-$C{{u}^{2+}}$lies below ${{H}^{+}}$within the table of ordinary reduction potentials.
-$C{{u}^{2+}}$is a stronger oxidant than ${{H}^{+}}$.
-$C{{u}^{2+}}$is more easily reduced than ${{H}^{+}}$.
-Keep in mind the impurities like Fe, Ni, Zn get dissolved within the solution but impurities like Au, Ag and Pt are deposited as anode mud below the anode.
Complete step by step answer:
Here we will take an example of electrolytic copper refining to understand the process more clearly. Copper is typically mined from its coal referred to as copper. It is about 98 to 99 percent pure. However, the electro-refining process can easily make it 99.95% pure which makes it a good product to be used in electrical components.
A block of impure copper is taken as an anode or positive electrode. Copper sulphate which is acidified with vitriol is employed as a graphite-coated electrolyte alongside pure copper tubes, as a cathode or negative electrode. In this phase of electrolysis copper sulphate divides into a positive ion of copper ($C{{u}^{2+}}$) and a negative ion of sulphate ($S{{O}_{4}}^{-}$). The positive copper ion ($C{{u}^{2+}}$) or cations travel towards the negative electrode made from pure copper where it absorbs the electrons from the cathode. Cu atoms are deposited on the cathode’s graphite layer.
The cathode is coated with graphite in the process of electrolytic metal processing or merely electro grinding so that the concentrated material can be easily removed. This is one of the most growing electrolysis procedures.
Electrolytic Refining of Copper

However, anode’s metallic impurities are also mixed with $S{{O}_{4}}$, forming metallic sulphate in the electrolyte solution and dissolving. In this above process, some impurities get dissolved in the solution while some deposits as anode mud below the anode electrode. Impurities which dissolve within the solution are Fe, Ni, Zn while impurities like Au, Ag and Pt deposits as anode mud below the anode electrode.
So, we can say that gold (Au) is deposited as Anode mud below the anode electrode.
Therefore, among the options if we check then option (C)Anode mud is the correct answer of the question.
Note:
- ${{H}^{+}}$from the electrolyte isn't reduced to ${{H}_{2}}$ (g) at the cathode.
-$C{{u}^{2+}}$lies below ${{H}^{+}}$within the table of ordinary reduction potentials.
-$C{{u}^{2+}}$is a stronger oxidant than ${{H}^{+}}$.
-$C{{u}^{2+}}$is more easily reduced than ${{H}^{+}}$.
-Keep in mind the impurities like Fe, Ni, Zn get dissolved within the solution but impurities like Au, Ag and Pt are deposited as anode mud below the anode.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




