
In order to convert Aniline into chlorobenzene, the reagents needed are:
(A) CuCl
(B) \[NaN{O_2}/HCl\] and CuCl
(C) \[C{l_2}/CC{l_4}\]
(D) \[C{l_2}/AlC{l_3}\]
Answer
233.1k+ views
Hint: This conversion can be brought by Sandmayer reaction. Here, we will also need to substitute the amino group from the ring by chlorine. So, whatever reagent we add during this reaction, should react with amino functional groups.
Complete Step-by-Step Solution:
- To convert Aniline into Chlorobenzene, we will need to remove the amino group and add chlorine atom to obtain chlorobenzene. There is only one reaction available that does this conversion.
- The name of this reaction is Sandmayer’s reaction. Let’s see the reaction to see the product obtained.
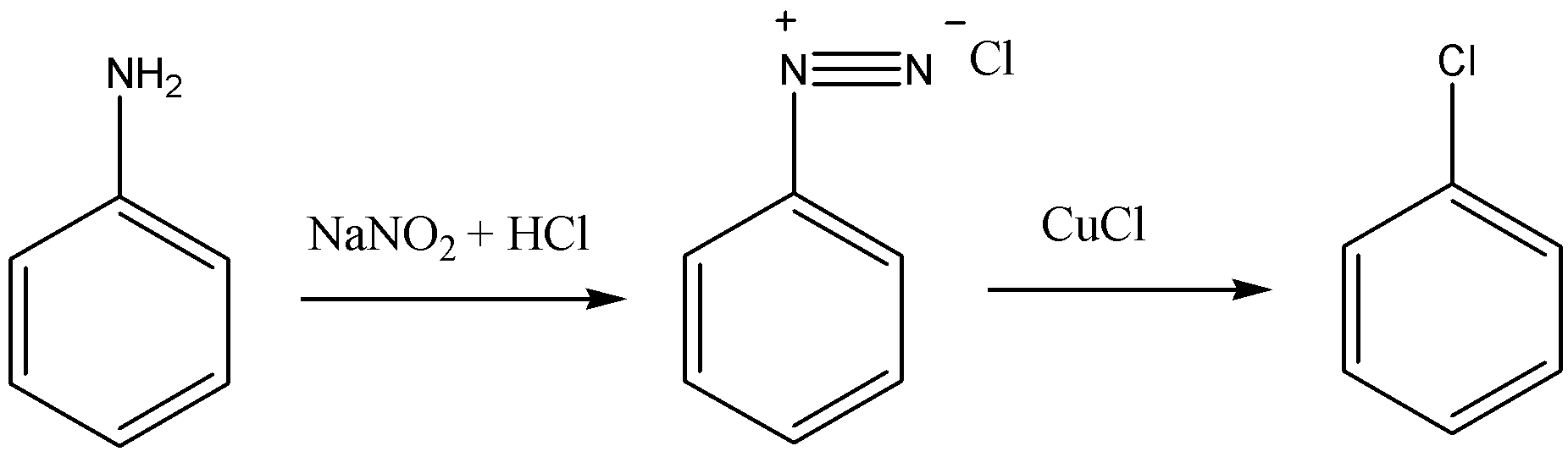
Sodium nitrite and hydrochloric acid react with aniline to form diazonium salt. Now, if this diazonium salt is allowed to react with CuCl, then it will replace the diazonium group on the aromatic ring and will give Chlorobenzene as a product.
- If we make aniline to react with just CuCl, then this reaction will not occur because diazonium salt will not be formed.
- \[C{l_2}/CC{l_4}\] is a reagent that substitutes chlorine atoms in place of hydrogen atoms of alkyl groups.
- If we will react \[C{l_2}/AlC{l_3}\] with aniline, then it will undergo Friedal-crafts reaction to give both amino and chlorine on the aromatic ring.
Hence, correct answer is (B) \[NaN{O_2}/HCl\] and CuCl
Additional Information:
- Many products can be obtained by reaction with diazonium salts. Below are some of them.
- If we add CuBr to diazonium salt then Bromobenzene will be the product.
- If CuCN is added then Cyanobenzene will be the product.
- If Water is added, then Phenol will be the product.
- If Hypophosphorous acid is added then, benzene will be the product.
Note: Remember that diazonium salt only gets converted to chlorobenzene when it is allowed to react with CuCl, so formation of diazonium salt is necessary. Do not get confused with \[C{l_2}/CC{l_4}\] as it does not substitute amino groups with chlorine atoms on aromatic rings.
Complete Step-by-Step Solution:
- To convert Aniline into Chlorobenzene, we will need to remove the amino group and add chlorine atom to obtain chlorobenzene. There is only one reaction available that does this conversion.
- The name of this reaction is Sandmayer’s reaction. Let’s see the reaction to see the product obtained.

Sodium nitrite and hydrochloric acid react with aniline to form diazonium salt. Now, if this diazonium salt is allowed to react with CuCl, then it will replace the diazonium group on the aromatic ring and will give Chlorobenzene as a product.
- If we make aniline to react with just CuCl, then this reaction will not occur because diazonium salt will not be formed.
- \[C{l_2}/CC{l_4}\] is a reagent that substitutes chlorine atoms in place of hydrogen atoms of alkyl groups.
- If we will react \[C{l_2}/AlC{l_3}\] with aniline, then it will undergo Friedal-crafts reaction to give both amino and chlorine on the aromatic ring.
Hence, correct answer is (B) \[NaN{O_2}/HCl\] and CuCl
Additional Information:
- Many products can be obtained by reaction with diazonium salts. Below are some of them.
- If we add CuBr to diazonium salt then Bromobenzene will be the product.
- If CuCN is added then Cyanobenzene will be the product.
- If Water is added, then Phenol will be the product.
- If Hypophosphorous acid is added then, benzene will be the product.
Note: Remember that diazonium salt only gets converted to chlorobenzene when it is allowed to react with CuCl, so formation of diazonium salt is necessary. Do not get confused with \[C{l_2}/CC{l_4}\] as it does not substitute amino groups with chlorine atoms on aromatic rings.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




