
In the experiment on the photoelectric effect, the graph between \[\mathop E\nolimits_K (\max )\] and \[\nu \] is found to be a straight line as shown in fig. The threshold frequency and Planck's constant according to this graph are:
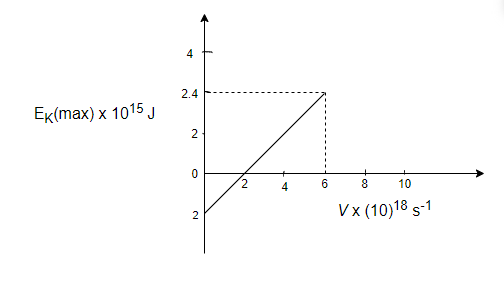
A) \[3.33 \times \mathop {10}\nolimits^{18} \]$s^{-1}$, \[6 \times \mathop {10}\nolimits^{ - 34} \]J-s
B) \[6 \times \mathop {10}\nolimits^{18} \] $s^{-1}$, \[6 \times \mathop {10}\nolimits^{ - 34} \] J-s
C) \[2.66 \times \mathop {10}\nolimits^{18} \] $s^{-1}$, \[4 \times \mathop {10}\nolimits^{ - 34} \] J-s
D) \[4 \times \mathop {10}\nolimits^{18} \] $s^{-1}$, \[3 \times \mathop {10}\nolimits^{ - 34} \] J-s
Answer
232.5k+ views
Hint: This given problem can be solved by Einstein’s photoelectric equation. Einstein explained the various laws of photoelectric emission on the basis of Planck’s quantum theory. According to Planck’s quantum theory, the energy of a photon is given by \[E = h\nu \].
Complete step by step solution:
Step 1:
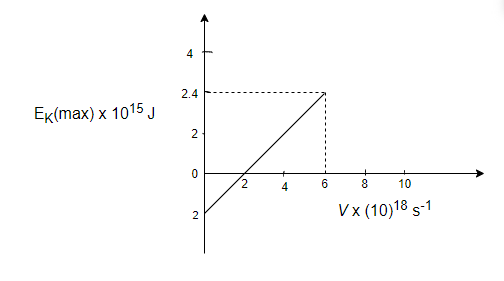
Einstein assumed that one photoelectron is ejected from a metal surface if one photon of suitable light radiation falls on it.
Let us consider a photon of light of frequency \[\nu \], incident on a photosensitive metal surface. The energy of the photon is \[h\nu \], spent in two ways:
A part of the energy of the photon will be used in liberating the electron from the metal surface which is equal to the work function \[\mathop \phi \nolimits_0 \] of the metal.
The rest of the energy of the photon will be used in imparting the maximum kinetic energy \[\mathop K\nolimits_{\max } \] to the emitted photoelectron.
So, from above two points, we will get-
\[h\nu = \mathop \phi \nolimits_0 + \mathop K\nolimits_{\max } \].................(1)
This equation (1) is known as Einstein’s photoelectric equation.
Step 2: Now from the equation (1) –
\[h\nu = \mathop \phi \nolimits_0 + \mathop K\nolimits_{\max } \]; where \[\mathop \phi \nolimits_0 = \]work function of metal, \[h = \]Planck’s constant, and \[\nu = \]frequency of incident photon
If we rearrange the equation (1) in the form of \[\mathop K\nolimits_{\max } = h\nu + \mathop \phi \nolimits_0 \] and this equation can be compared with the \[y = mx + c\] .
So, the slope of the given line will be \[h\] and the intercept will be \[ - \mathop \phi \nolimits_0 \].
So, from the graph, \[h = \dfrac{{\mathop K\nolimits_{\max } }}{{\mathop \nu \nolimits_2 - \mathop \nu \nolimits_1 }}\]
\[h = \dfrac{{2.4 \times \mathop {10}\nolimits^{ - 15} }}{{\left( {6 - 2} \right) \times \mathop {10}\nolimits^{18} }}\]
\[h = 6 \times \mathop {10}\nolimits^{ - 34} \]J-s
And \[\mathop \phi \nolimits_0 = 2 \times \mathop {10}\nolimits^{ - 15} \]J
\[\mathop \phi \nolimits_0 = h\mathop \nu \nolimits_0 \]; where \[\mathop \nu \nolimits_0 = \] threshold frequency
\[\mathop \nu \nolimits_0 = \dfrac{{\mathop \phi \nolimits_0 }}{h} = \dfrac{{2 \times \mathop {10}\nolimits^{ - 15} }}{{6 \times \mathop {10}\nolimits^{ - 34} }}\]
\[\mathop \nu \nolimits_0 = 3.33 \times \mathop {10}\nolimits^{18} \]$s^{-1}$
So, the correct option is (A).
Note: If \[\nu < \mathop \nu \nolimits_0 \] , then maximum K.E. will be negative, which is not possible. So, Photoelectric emission does not occur if the frequency of incident radiation is less than the threshold frequency.
Complete step by step solution:
Step 1:
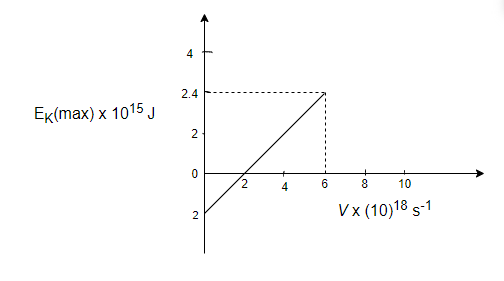
Einstein assumed that one photoelectron is ejected from a metal surface if one photon of suitable light radiation falls on it.
Let us consider a photon of light of frequency \[\nu \], incident on a photosensitive metal surface. The energy of the photon is \[h\nu \], spent in two ways:
A part of the energy of the photon will be used in liberating the electron from the metal surface which is equal to the work function \[\mathop \phi \nolimits_0 \] of the metal.
The rest of the energy of the photon will be used in imparting the maximum kinetic energy \[\mathop K\nolimits_{\max } \] to the emitted photoelectron.
So, from above two points, we will get-
\[h\nu = \mathop \phi \nolimits_0 + \mathop K\nolimits_{\max } \].................(1)
This equation (1) is known as Einstein’s photoelectric equation.
Step 2: Now from the equation (1) –
\[h\nu = \mathop \phi \nolimits_0 + \mathop K\nolimits_{\max } \]; where \[\mathop \phi \nolimits_0 = \]work function of metal, \[h = \]Planck’s constant, and \[\nu = \]frequency of incident photon
If we rearrange the equation (1) in the form of \[\mathop K\nolimits_{\max } = h\nu + \mathop \phi \nolimits_0 \] and this equation can be compared with the \[y = mx + c\] .
So, the slope of the given line will be \[h\] and the intercept will be \[ - \mathop \phi \nolimits_0 \].
So, from the graph, \[h = \dfrac{{\mathop K\nolimits_{\max } }}{{\mathop \nu \nolimits_2 - \mathop \nu \nolimits_1 }}\]
\[h = \dfrac{{2.4 \times \mathop {10}\nolimits^{ - 15} }}{{\left( {6 - 2} \right) \times \mathop {10}\nolimits^{18} }}\]
\[h = 6 \times \mathop {10}\nolimits^{ - 34} \]J-s
And \[\mathop \phi \nolimits_0 = 2 \times \mathop {10}\nolimits^{ - 15} \]J
\[\mathop \phi \nolimits_0 = h\mathop \nu \nolimits_0 \]; where \[\mathop \nu \nolimits_0 = \] threshold frequency
\[\mathop \nu \nolimits_0 = \dfrac{{\mathop \phi \nolimits_0 }}{h} = \dfrac{{2 \times \mathop {10}\nolimits^{ - 15} }}{{6 \times \mathop {10}\nolimits^{ - 34} }}\]
\[\mathop \nu \nolimits_0 = 3.33 \times \mathop {10}\nolimits^{18} \]$s^{-1}$
So, the correct option is (A).
Note: If \[\nu < \mathop \nu \nolimits_0 \] , then maximum K.E. will be negative, which is not possible. So, Photoelectric emission does not occur if the frequency of incident radiation is less than the threshold frequency.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students

Understanding Electromagnetic Waves and Their Importance




