
Iodoform can be prepared from all except:
A. Acetaldehyde
B. \[3 - \]methyl\[ - 2 - \]butanone
C. Isobutyl alcohol
D. Acetophenone
Answer
232.8k+ views
Hint: Iodoform is an organoiodine compound with the formula \[{\text{CH}}{{\text{I}}_3}\].
We can synthesize iodoform using organic compounds having aldehyde or ketone group i.e carbonyl group having a methyl group on the side.
Complete step by step answer:
We can perform iodoform reaction using any of these four kinds of organic compounds \[i.e\]. a Methyl ketone (\[{\text{C}}{{\text{H}}_3}{\text{COR}}\]) or acetaldehyde (\[{\text{C}}{{\text{H}}_3}{\text{CHO}}\]) or ethanol (\[{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}\]) or secondary alcohol. As all of the above mentioned organic compounds contain group or group.
Now, we will see the reaction scheme of iodoform reaction.
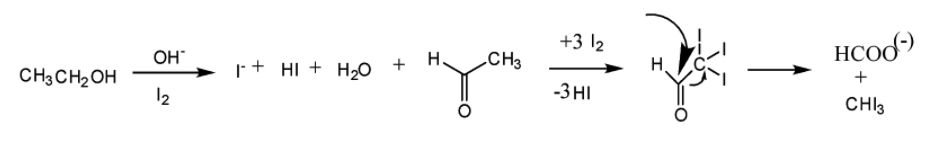
So, from the above discussion, we understand that the compound having an aldehydic group or -methyl ketone group will give this reaction.
Now, we will see the structure of the given compound.
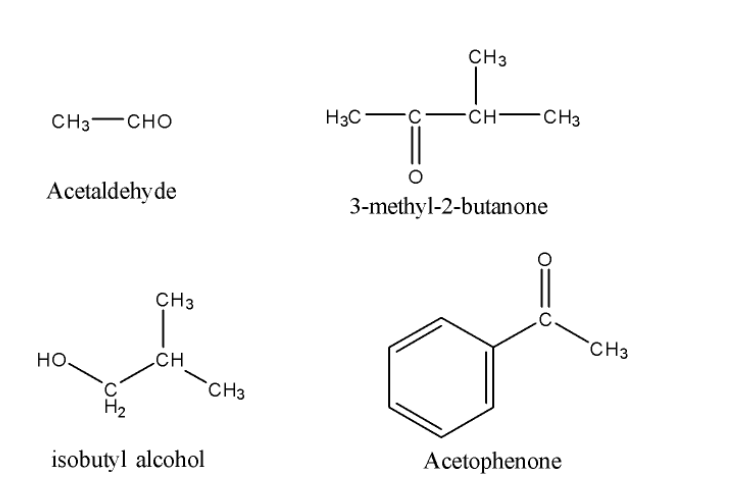
So, from the above structures, we can easily find out that all compounds except Isobutyl alcohol are having the required groups mentioned in the hint i.e aldehydic group or ketonic group with methyl on the side.
So, only the isobutyl alcohol will not give response to the iodoform test.
So, Iodoform can be prepared from all except isobutyl alcohol.
So, the correct option is option\[ - \left( {\mathbf{C}} \right).\]
Note:
1. Iodoform is a pale yellow colored, volatile substance and has a distinctive odor.
2. Like iodoform, there are chloroform and bromoform also. All the reactions go via the same mechanism.
3. While performing the iodoform reaction, care must be taken because of the corrosiveness of iodine.
We can synthesize iodoform using organic compounds having aldehyde or ketone group i.e carbonyl group having a methyl group on the side.
Complete step by step answer:
We can perform iodoform reaction using any of these four kinds of organic compounds \[i.e\]. a Methyl ketone (\[{\text{C}}{{\text{H}}_3}{\text{COR}}\]) or acetaldehyde (\[{\text{C}}{{\text{H}}_3}{\text{CHO}}\]) or ethanol (\[{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}\]) or secondary alcohol. As all of the above mentioned organic compounds contain group or group.
Now, we will see the reaction scheme of iodoform reaction.

So, from the above discussion, we understand that the compound having an aldehydic group or -methyl ketone group will give this reaction.
Now, we will see the structure of the given compound.

So, from the above structures, we can easily find out that all compounds except Isobutyl alcohol are having the required groups mentioned in the hint i.e aldehydic group or ketonic group with methyl on the side.
So, only the isobutyl alcohol will not give response to the iodoform test.
So, Iodoform can be prepared from all except isobutyl alcohol.
So, the correct option is option\[ - \left( {\mathbf{C}} \right).\]
Note:
1. Iodoform is a pale yellow colored, volatile substance and has a distinctive odor.
2. Like iodoform, there are chloroform and bromoform also. All the reactions go via the same mechanism.
3. While performing the iodoform reaction, care must be taken because of the corrosiveness of iodine.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




