
Nitrene is an intermediate in one of the following reactions:
A. Schmidt rearrangement
B. Beckmann rearrangement
C. Baeyer-Villiger oxidation
D. Curtius reaction
Answer
232.8k+ views
Hint : Nitrene is the nitrogen analogue of a carbene. It is an electrophile because it has five valence electrons. It is involved in many chemical reactions and acts as a reactive intermediate. It can be generated by two common ways. It can be generated by thermolysis or photolysis of azides with expulsion of nitrogen gas. Another way of generation of nitrene is the expulsion of carbon monoxide.
Complete step by step solution:
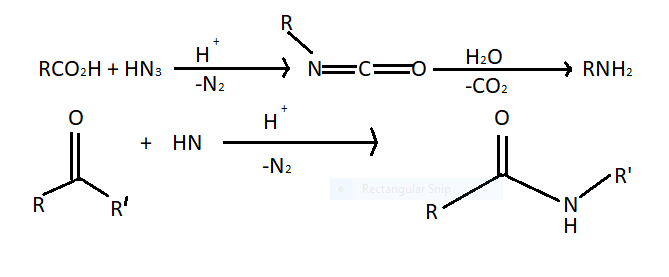
In Schmidt rearrangement alkyl migration over carbon nitrogen bonds takes place. This reaction refers to a reaction between an acid catalyst hydrazoic acid with electrophiles. Carboxylic acid forms amines through an isocyanate intermediate and ketones from amides. Nitrene is an intermediate in the following reactions.
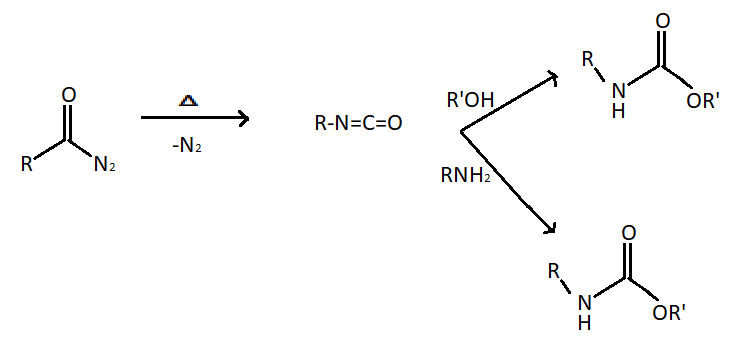
In the Curtius reaction, the production of an isocyanate is accomplished by the thermal decomposition of carboxylic azides. The mechanism involves the alkyl shift of the $R$ group from carbonyl carbon to the closet nitrogen. The reaction has similarities with Schmidt reaction with acids but differs in that the acyl azide in the present case is prepared from acyl halide and an azide salt.
Hence option A and D both are correct answers of this problem because in both of the reactions nitrene is an intermediate.
Note : Nitrenes are highly reactive molecule species with a monovalent nitrogen atom which can exist in a singlet and triplet state. Nitrenes are more stable than carbene. It is because of greater thermodynamic stability of nitrene. As nitrenes are very reactive they are not isolated. The insertion of nitrene can be easily into a carbon to hydrogen covalent bond yielding an amine or amide.
Complete step by step solution:
In Schmidt rearrangement alkyl migration over carbon nitrogen bonds takes place. This reaction refers to a reaction between an acid catalyst hydrazoic acid with electrophiles. Carboxylic acid forms amines through an isocyanate intermediate and ketones from amides. Nitrene is an intermediate in the following reactions.

In the Curtius reaction, the production of an isocyanate is accomplished by the thermal decomposition of carboxylic azides. The mechanism involves the alkyl shift of the $R$ group from carbonyl carbon to the closet nitrogen. The reaction has similarities with Schmidt reaction with acids but differs in that the acyl azide in the present case is prepared from acyl halide and an azide salt.

Hence option A and D both are correct answers of this problem because in both of the reactions nitrene is an intermediate.
Note : Nitrenes are highly reactive molecule species with a monovalent nitrogen atom which can exist in a singlet and triplet state. Nitrenes are more stable than carbene. It is because of greater thermodynamic stability of nitrene. As nitrenes are very reactive they are not isolated. The insertion of nitrene can be easily into a carbon to hydrogen covalent bond yielding an amine or amide.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




