
Number of \[\pi \]- electrons in cyclobutadienyl anion ${{\text{(}{{\text{C}}_{4}}{{\text{H}}_{4}})}^{-2}}$ is:
(A) 2
(B) 4
(C) 6
(D) 8
Answer
233.1k+ views
Hint: For this problem, we have to understand the concept of pi - electrons first. And then we have to draw the proper diagram of cyclobutadienyl through which can easily count the total number of pi - electrons.
Complete step by step solution:
-In the given question we have to calculate the total number of pi - electrons which are present in the cyclobutadienyl anion.
-So, firstly we should know about the pi - electrons. As we know that two kinds of electrons are sigma and pi electrons.
-So, pi electrons are the type of electrons which are present in the pi - bond such as double and triple bond whereas sigma electrons are found in sigma bonds such as a single bond.
-Now, to calculate the total number of pi - electrons firstly we have to draw the structure of cyclobutadienyl anion that is:
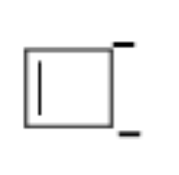
-So, as we can see there is one double bond which means 2 pi electrons because we know that the one bond consists of two electrons and along with it two negative charges are also present.
-We know that each charge consists of a total of two electrons so a total of 4 pi - electrons will be present.
-Thus, there are a total of 6 pi - electrons present in cyclobutadienyl anion.
-And a total of 4 sigma bonds or 8 sigma electrons are present in the cyclobutadienyl anion.
Therefore, option (C) is the correct answer.
Note: The sigma and pi bond are different from each other due to the different types of overlapping because the sigma bond is formed from the head-on overlapping whereas pi bond is formed from lateral overlapping.
Complete step by step solution:
-In the given question we have to calculate the total number of pi - electrons which are present in the cyclobutadienyl anion.
-So, firstly we should know about the pi - electrons. As we know that two kinds of electrons are sigma and pi electrons.
-So, pi electrons are the type of electrons which are present in the pi - bond such as double and triple bond whereas sigma electrons are found in sigma bonds such as a single bond.
-Now, to calculate the total number of pi - electrons firstly we have to draw the structure of cyclobutadienyl anion that is:

-So, as we can see there is one double bond which means 2 pi electrons because we know that the one bond consists of two electrons and along with it two negative charges are also present.
-We know that each charge consists of a total of two electrons so a total of 4 pi - electrons will be present.
-Thus, there are a total of 6 pi - electrons present in cyclobutadienyl anion.
-And a total of 4 sigma bonds or 8 sigma electrons are present in the cyclobutadienyl anion.
Therefore, option (C) is the correct answer.
Note: The sigma and pi bond are different from each other due to the different types of overlapping because the sigma bond is formed from the head-on overlapping whereas pi bond is formed from lateral overlapping.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




