




How Are Nuclear Fission and Fusion Different?
Nuclear fission and fusion are fundamental nuclear reactions in physics, where large amounts of energy are released by changing the configuration of atomic nuclei. These processes are significant for understanding both natural phenomena and technological applications in energy generation and astrophysics.
Nuclear Fission: Principle and Process
Nuclear fission is a process in which a heavy atomic nucleus splits into two or more lighter nuclei, accompanied by the release of energy and additional neutrons. This reaction is generally initiated when a nucleus such as uranium-235 or plutonium-239 absorbs a low-energy neutron, resulting in an unstable compound nucleus that undergoes division.
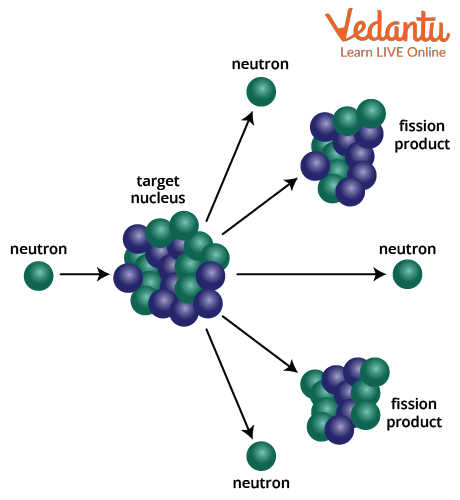
The typical fission reaction for uranium-235 can be represented as:
$\displaystyle {}_{92}^{235}U+{}_{0}^{1}n\rightarrow {}_{56}^{141}Ba+{}_{36}^{92}Kr+3{}_{0}^{1}n$
The large energy release in fission arises due to the difference in binding energies of the parent and product nuclei. The fission products are often radioactive and can pose environmental challenges. To learn more about nuclear structure and its importance in fission, refer to Nuclear Structure, Composition, And Size.
Nuclear Fusion: Fundamentals and Mechanism
Nuclear fusion involves the combination of two light atomic nuclei to form a single heavier nucleus. This fusion process requires extremely high temperatures and pressures to overcome electrostatic repulsion between the positively charged nuclei.
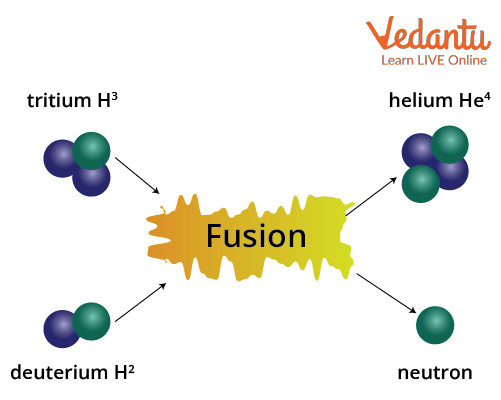
A common example of fusion is the reaction between two deuterium nuclei:
$\displaystyle {}_{1}^{2}H+{}_{1}^{2}H\rightarrow {}_{2}^{3}He+{}_{0}^{1}n$
Fusion reactions release more energy per nucleon than fission and occur naturally in stars, including the Sun. The fusion of deuterium and tritium nuclei (${}_{1}^{2}H+{}_{1}^{3}H$) is utilized in research on harnessing thermonuclear energy.
Fusion requires conditions where nuclei possess kinetic energies sufficiently high to bring them close enough for nuclear forces to take effect. These processes are also relevant for understanding stellar structures, as explained in the context of Uses Of Spherical Mirrors, which are essential in optical diagnostics of plasma in fusion research.
Mathematical Analysis and Energy Release
The energy released in nuclear reactions is given by Einstein's equation: $E = \Delta m c^2$, where $\Delta m$ is the mass defect and $c$ is the speed of light. In both fission and fusion, the total mass of the products is less than that of the reactants, and the difference is converted into energy.
In fission, each Uranium-235 event typically releases about 200 MeV of energy. In fusion, the deuterium-tritium reaction releases about 17.6 MeV, but fusion is substantially more energy-dense per unit mass due to the low mass and high abundance of participating nuclei. For detailed understanding of nuclear decay processes related to these reactions, see Alpha, Beta, And Gamma Decay.
Natural and Artificial Occurrence
Nuclear fission can occur naturally in uranium deposits, but is mostly controlled in nuclear reactors. Fusion occurs naturally in stars, powering the Sun and enabling life on Earth. Artificial attempts at fusion include magnetic confinement in Tokamaks and inertial confinement methods.
Fission can initiate a chain reaction when the neutrons produced create further fission events. Fusion reactions, however, do not naturally self-sustain under terrestrial conditions due to challenging temperature and pressure requirements.
Comparison Table: Nuclear Fission and Fusion
| Nuclear Fission | Nuclear Fusion |
|---|---|
| Heavy nucleus splits into lighter nuclei | Light nuclei combine to form heavier nucleus |
| Initiated by neutron absorption | Requires high temperature and pressure |
| Occurs in nuclear reactors and weapons | Occurs in stars and hydrogen bombs |
| Produces radioactive waste | Generally no long-lived radioactive waste |
| Relatively lower energy per nucleon | Higher energy per nucleon |
| Chain reaction possible and controllable | Chain reaction not feasible naturally on earth |
Applications of Fission and Fusion
Nuclear fission is utilized in nuclear power plants, where the heat generated is used to produce steam for electricity generation. Fission is also the basis of atomic bombs. Nuclear fusion, if controlled on Earth, has the potential for abundant clean energy and is already responsible for solar energy.
Fusion reactions are being researched extensively for future energy needs due to their advantages, such as the absence of greenhouse gas emissions and minimal long-lived nuclear waste. Current fission technology is advanced and commercialized, while controlled fusion is still under development. For details on reactors, refer to Nuclear Fission And Nuclear Reactor.
Key Differences: Fission vs. Fusion
- Fission splits heavy atoms; fusion merges light atoms
- Fission requires critical mass; fusion requires high temperature
- Fission produces radioactive waste; fusion does not
- Fission is technologically mature; fusion is under research
- Fission can be controlled in reactors; fusion control is challenging
Significance in Physics and Energy
Both nuclear fission and fusion have deep significance in physics for understanding atomic interactions, binding energy, and energy transformation. They represent practical routes for energy generation, each presenting unique safety and environmental considerations.
The study of these nuclear reactions is integral to modern physics and forms the basis for understanding radioactivity, decay laws, and energy production. For an in-depth look at decay processes, refer to Law Of Radioactive Decay.
The mastery of fission and fusion topics is essential for JEE aspirants as it connects several important theoretical and application-based concepts in modern physics. For complete details and further learning on nuclear reactions, visit Nuclear Fission And Fusion.
FAQs on Understanding Nuclear Fission and Fusion
1. What is nuclear fission?
Nuclear fission is the process where a heavy nucleus, such as uranium-235 or plutonium-239, splits into two lighter nuclei, releasing a large amount of energy.
Key facts:
- Occurs when a nucleus absorbs a neutron and becomes unstable.
- Results in the release of neutrons and energy in the form of heat and radiation.
- This principle is used in nuclear reactors for power generation and in atomic bombs for explosions.
2. What is nuclear fusion, and how does it differ from fission?
Nuclear fusion is the process where two light nuclei combine to form a single, heavier nucleus, releasing a tremendous amount of energy.
Differences include:
- Fusion involves merging of small nuclei (e.g., hydrogen isotopes), while fission splits heavy nuclei.
- Fusion produces much more energy per reaction than fission.
- Fusion is the source of energy in stars, including the sun.
3. What are the main differences between nuclear fission and nuclear fusion?
The main differences between nuclear fission and nuclear fusion are based on their process, fuel, and byproducts.
Key distinctions:
- Fission splits heavy nuclei; Fusion joins light nuclei.
- Fission produces radioactive waste; fusion produces less or no radioactive waste.
- Fusion requires extremely high temperature and pressure; fission can happen at lower temperatures with a neutron.
- Both release energy, but fusion generates more energy than fission.
4. Where does nuclear fusion occur naturally?
Nuclear fusion occurs naturally in the core of stars, including our sun.
Main points:
- Stars fuse hydrogen nuclei to form helium via the fusion process.
- This fusion reaction releases enormous amounts of energy as heat and light.
- Fusion powers all life on Earth by providing sunlight.
5. What are the uses of nuclear fission?
The primary use of nuclear fission is to generate electricity in nuclear power plants.
Other uses include:
- Production of nuclear weapons.
- Creation of medical isotopes for diagnosis and treatment.
- Research purposes in scientific laboratories.
6. Why is nuclear fusion considered cleaner than nuclear fission?
Nuclear fusion is considered cleaner because it produces significantly less radioactive waste than fission and uses abundant fuels like hydrogen.
Key reasons:
- No long-lived radioactive byproducts.
- Lower risks of environmental contamination.
- Fusion fuel (like hydrogen) is easily available from water.
7. What is a chain reaction in nuclear fission?
A chain reaction in nuclear fission occurs when released neutrons from one fission event cause further fission reactions.
Key aspects:
- It sustains and multiplies energy release.
- Controlled chain reactions are used in nuclear reactors.
- Uncontrolled chain reactions cause nuclear explosions.
8. What are some challenges in achieving nuclear fusion on Earth?
The primary challenges in achieving nuclear fusion are reaching and maintaining extremely high temperatures and pressures required for fusion to occur.
Main obstacles:
- Need for temperatures over 100 million degrees Celsius.
- Containment of hot plasma, often with magnetic fields (tokamaks, stellarators).
- Current fusion reactors consume more energy than they produce.
9. What are the advantages and disadvantages of nuclear fission?
Nuclear fission offers both benefits and risks as an energy source.
Advantages:
- High energy output from a small amount of fuel.
- No greenhouse gas emissions during operation.
- Production of harmful radioactive waste.
- Risk of nuclear accidents and misuse in weapons.
10. Explain energy generation in a nuclear reactor using fission.
In a nuclear reactor, controlled fission reactions of fuel (like uranium-235) produce heat energy.
Key steps:
- Neutrons split heavy nuclei, releasing heat and more neutrons.
- Heat is absorbed by coolant.
- Steam generated drives turbines, producing electricity.
11. What is meant by critical mass in nuclear fission?
Critical mass is the minimum amount of fissile material needed to maintain a self-sustaining nuclear chain reaction.
Key points:
- If the mass is below critical, the reaction stops.
- Used to safely design nuclear reactors and weapons.
12. How is nuclear fusion useful for future energy needs?
Nuclear fusion holds promise as a clean, abundant, and sustainable source of energy for the future.
Reasons include:
- Unrestricted fuel supply (hydrogen from water).
- No greenhouse gases or long-lasting radioactive waste.
- High energy yield can meet growing electricity demands.
























