




How Do Solids Affect Surface Tension?
Solids and surface tension play a crucial role in understanding forces at material boundaries for JEE and NEET Physics aspirants.
Surface tension describes the elastic-like tendency of a liquid’s surface, causing it to behave as if under a stretched, invisible membrane.
In daily life, water droplets on a leaf or the ability of a needle to float showcase the remarkable effects of surface tension.
What is Surface Tension?
Surface tension is the force that causes a liquid’s surface to contract, minimizing its surface area due to cohesive molecular interactions at the interface.
This phenomenon results because molecules at the surface experience unbalanced forces, attracting them more strongly toward the interior than toward the air.
Why Surface Tension Occurs: Real-Life Visualisation
Imagine water molecules in a drop; those in the bulk are pulled equally in all directions, but surface molecules are pulled inward, causing minimal surface area.
The iconic rounded shape of water drops and even the formation of soap bubbles are direct results of this inward pulling force.
Surface Tension: Formula, Unit, and Dimensions
Mathematically, surface tension $T$ is defined as the force $F$ acting along a length $L$ at the surface: $T = \dfrac{F}{L}$.
The SI unit is Newton per metre (N/m), while the CGS unit is dyne/cm; in dimensions, surface tension is $[M T^{-2}]$.
| Parameter | Value/Unit |
|---|---|
| Surface Tension of Water (20°C) | 0.0728 N/m |
| SI Unit | N/m |
| CGS Unit | dyne/cm |
| Dimension | $[M T^{-2}]$ |
For detailed differences between the properties of solids and liquids, explore the Properties Of Solids And Liquids resource.
Surface Energy and Its Connection with Surface Tension
Surface energy is the work required to increase the surface area of a liquid by one unit, and it’s measured in J/m², numerically equal to surface tension for liquids.
When a surface is created, molecules at the interface break some intermolecular bonds, and this energy input is quantified as surface energy.
Do Solids Have Surface Tension?
For solids, especially crystalline materials, the concept similar to liquid surface tension exists but is called surface free energy per unit area.
Unlike liquids, solid surfaces can support shear stresses, so surface “tension” in solids involves a combination of mechanical and thermodynamic effects.
A major difference is that in liquids, surface tension and surface energy are numerically equal, but in solids, they differ due to anisotropy and structure.
Solid-Liquid-Gas Interface: The Contact Angle Concept
At the junction of solid, liquid, and gas, the meniscus formation and wetting behavior are governed by the balance of surface and interfacial tensions.
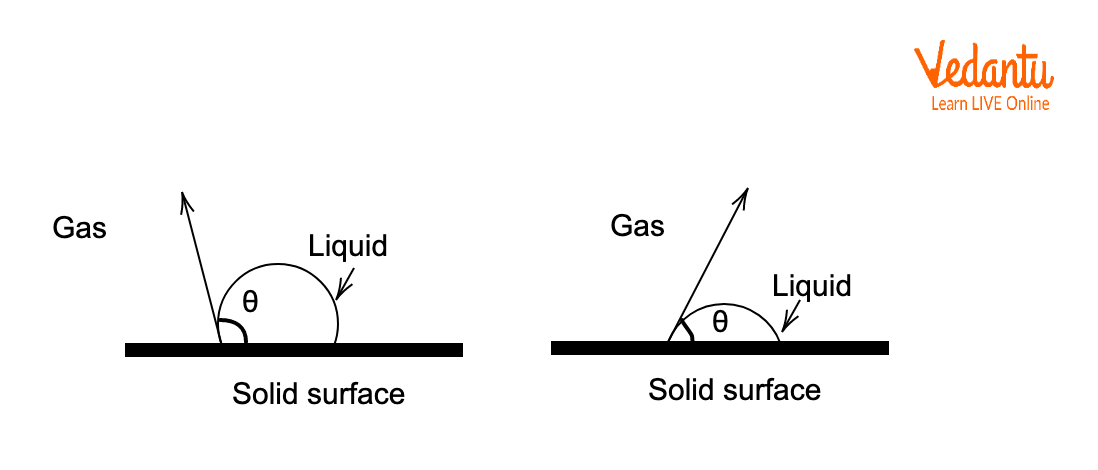
The contact angle $\theta$ quantifies wetting, and Young’s equation relates surface tensions as: $\gamma_{sg} = \gamma_{sl} + \gamma_{lg}\cos\theta$.
Where $\gamma_{sg}$, $\gamma_{sl}$, and $\gamma_{lg}$ are the solid-gas, solid-liquid, and liquid-gas tensions respectively; a small $\theta$ means better wetting.
Measuring Surface Tension: Laboratory Methods
Surface tension can be experimentally measured by techniques like the capillary rise method, drop weight method, and the use of stalagmometers or spinning drop devices.
- Capillary rise method
- Pendant drop method
- Stalagmometric technique
- Bubble pressure method
Explore advanced discussions of surface tension and contact angle in our detailed coverage: Surface Tension And Contact Angle.
Practical Applications of Surface Tension
Surface tension is why insects like water striders walk on water and why detergents can make water spread and clean more effectively.
- Floating of small objects
- Soap bubble formation
- Capillary action in plants
- Droplet formation in inkjet printers
- Emulsion stability in food processing
You can practice these properties further by using our Properties Of Solids And Liquids Practice Paper designed for students.
Surface Tension in Metals and Solids
Metals at their molten state exhibit measurable surface tension, e.g., mercury’s high value explains the nearly spherical drops you observe in laboratories.
In solids, atoms at the crystal surface have higher energy due to incomplete bonding, resulting in what is known as surface free energy.
| Material | Surface Tension (N/m) |
|---|---|
| Water (20°C) | 0.0728 |
| Mercury (25°C) | 0.485 |
| Molten Iron (1600°C) | 1.9 |
Surface Tension in JEE: Key Concepts and Problem Approach
JEE exam often asks to calculate forces on floating bodies, energy required to form bubbles, or the capillary rise observed in glass tubes.
- Deriving capillary rise using $h = \dfrac{2T \cos\theta}{\rho g r}$
- Numerical on maximum drop size supported by a wire frame
- Surface energy change during soap bubble expansion
Studying the link between thermodynamics and surface energy enhances conceptual understanding; refer to Thermodynamics for related insights.
Solved Example: JEE Level Numerical
A glass capillary of radius $0.5$ mm is placed in water ($T = 0.072$ N/m, $\theta = 0^\circ, \rho = 1000$ kg/m³, $g = 9.8$ m/s²). Find the capillary rise $h$.
Using $h = \dfrac{2T \cos\theta}{\rho g r}$:
$h = \dfrac{2 \times 0.072 \times 1}{1000 \times 9.8 \times 0.0005} = \dfrac{0.144}{4.9} \approx 0.029$ m $= 2.9$ cm.
Practice Question
The surface tension of liquid A is twice that of liquid B. A capillary rises $2$ cm in A; what is the rise in B under identical conditions?
Common Mistakes to Avoid
- Confusing surface tension and surface energy for solids
- Ignoring the effect of temperature on surface tension
- Incorrectly applying capillary formulas with the wrong angle $\theta$
Concepts of thermal variations and expansion can be further studied via Thermal Expansion.
Related Topics Worth Exploring
- Molecular structure of solids and liquids
- Elastic properties of materials
- Surface tension in biological fluids
- Capillarity and its engineering applications
- Free energy concepts in materials science
FAQs on Understanding Solids and Surface Tension
1. What is surface tension in solids and liquids?
Surface tension is the property of the surface of a liquid or solid that allows it to resist an external force, due to cohesive forces among its molecules.
Key points:
- In liquids, surface tension is responsible for phenomena like droplets forming and insects walking on water.
- In solids, the concept relates to surface energy and the tendency for solids to minimize their surface area.
2. What is meant by adhesion and cohesion?
Cohesion is the force of attraction between molecules of the same substance, while adhesion is the attraction between molecules of different substances.
For example:
- Cohesion helps water molecules stick together (surface tension).
- Adhesion allows water to cling to glass or plant walls (capillary action).
3. Explain capillary action with an example.
Capillary action is the ability of a liquid to flow in narrow spaces without external forces, due to surface tension and adhesion.
Examples:
- Water rising in a thin glass tube.
- Uptake of water by the roots of plants.
4. How does surface tension affect the shape of a liquid drop?
Surface tension causes a liquid drop to acquire a spherical shape as this minimizes the surface area for a given volume.
Key points:
- Sphere has the smallest surface area.
- Molecules are pulled inward equally by cohesive forces.
5. What factors influence the surface tension of a liquid?
Surface tension depends on several factors:
- Nature of the liquid (e.g., water vs. alcohol).
- Temperature (surface tension decreases with increasing temperature).
- Presence of impurities (e.g., soaps and detergents lower water's surface tension).
6. State the applications of surface tension in daily life.
Surface tension has various daily life applications:
- Cleaning and washing (detergents reduce water's surface tension).
- Formation of droplets (rain, ink on paper).
- Floating of small insects on water.
- Capillary rise in plant stems for water absorption.
7. Give two ways of increasing or decreasing the surface tension of a liquid.
Surface tension can be changed by:
- Heating: Increases temperature and decreases surface tension.
- Adding surfactants (like soap): Decreases surface tension.
- Cooling: Lowers temperature and increases surface tension.
8. What is the effect of temperature on surface tension?
Raising the temperature of a liquid decreases its surface tension.
Reasons:
- Increased molecular movement reduces cohesive forces.
- Higher temperature causes molecules to move apart, weakening attractions.
9. What is meant by surface energy in solids?
Surface energy is the energy required to create a unit area of surface in a solid.
Details:
- It arises due to unsatisfied bonds at the surface compared to the bulk interior.
- Determines properties like wetting, adhesion, and fracture strength.
10. Explain the difference between surface tension and surface energy.
Surface tension is a measurable force along a liquid's surface; surface energy is energy per unit area associated with a solid's surface.
Differences:
- Surface tension is for liquids; surface energy is for solids (and sometimes liquids).
- Surface tension measured in N/m, surface energy in J/m².
11. Why do insects like mosquitoes float on water?
Insects such as mosquitoes float on water due to surface tension, which creates a "skin" strong enough to support their weight.
Details:
- Insects spread their weight over a larger area.
- Legs are hydrophobic, preventing them from breaking the water surface.
12. How does the contact angle relate to surface tension and wetting of solids?
Contact angle is the angle at which a liquid interface meets a solid surface, showing how well a liquid wets the surface.
Key points:
- Small contact angle (less than 90°): Good wetting, strong adhesion.
- Large contact angle (greater than 90°): Poor wetting, weak adhesion.
- Depends on surface tension and surface energy of both liquid and solid.
























