
The monomer used to produce orlon is:
(A) \[C{{H}_{2}}=CHF\]
(B) \[C{{H}_{2}}=CC{{l}_{2}}\]
(C) \[C{{H}_{2}}=CHCl\]
(D) \[C{{H}_{2}}=CHCN\]
Answer
233.1k+ views
Hint:
Orlon is also known as polyacrylonitrile. As the name contains a nitrile term it suggests that N is present in the monomer. According to this we can directly conclude D as a correct answer.
Complete step by step answer:
> Monomers are small molecules, mostly organic, that can join with other similar molecules to form very large molecules, or polymers. All monomers have the capacity to form chemical bonds to at least two other monomer molecules.
> Polymers are a class of synthetic substances composed of multiples of simpler units called monomers. Polymers are chains with an unspecified number of monomeric units.
> Orlon is a polyacrylonitrile. It is an additional polymer. The addition of polymerisation of acrylonitrile in presence of a peroxide catalyst leads to the formation of polyacrylonitrile.
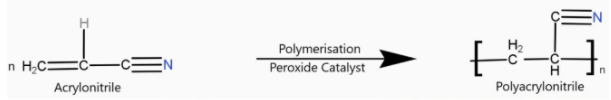
Polyacrylonitrile is used as a substitute for wool in making commercial fibres as orlon or acrilan.
Hence, the monomer used to produce orlon is Acrylonitrile (vinyl cyanide) $C{{H}_{2}}=CHCN$.
So, the correct option is (D).
Note:
Monomers are small molecules that can join with other similar molecules to form very large molecules, or polymers. Remember, Orlon is known as Polyacrylonitrile(PAN).
Orlon is also known as polyacrylonitrile. As the name contains a nitrile term it suggests that N is present in the monomer. According to this we can directly conclude D as a correct answer.
Complete step by step answer:
> Monomers are small molecules, mostly organic, that can join with other similar molecules to form very large molecules, or polymers. All monomers have the capacity to form chemical bonds to at least two other monomer molecules.
> Polymers are a class of synthetic substances composed of multiples of simpler units called monomers. Polymers are chains with an unspecified number of monomeric units.
> Orlon is a polyacrylonitrile. It is an additional polymer. The addition of polymerisation of acrylonitrile in presence of a peroxide catalyst leads to the formation of polyacrylonitrile.

Polyacrylonitrile is used as a substitute for wool in making commercial fibres as orlon or acrilan.
Hence, the monomer used to produce orlon is Acrylonitrile (vinyl cyanide) $C{{H}_{2}}=CHCN$.
So, the correct option is (D).
Note:
Monomers are small molecules that can join with other similar molecules to form very large molecules, or polymers. Remember, Orlon is known as Polyacrylonitrile(PAN).
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




