
The monomers of biodegradable polymer, nylon 2-nylon 6 are:
A. glycine + adipic acid
B. glycol + phthalic acid
C. phenol + urea
D. glycine + amino caproic acid
Answer
233.1k+ views
Hint: Nylon 2-nylon 6 is a condensation polymer. It is a result of polyamide copolymerization of two monomers, one has both amino group and carboxylic acid and another is a derivative of amino acid lysine.
Complete step by step answer:
Polymerization is a process in which small molecules, known as monomers combine chemically to produce a very large network molecule which is called a polymer.
Monomers are the molecules that are bonded to other molecules to form a polymer.
There are two monomers of Nylon 2-nylon 6 named as Glycine $\left( \text{Gly} \right)$ with chemical formula $\left( {{\text{H}}_{2}}\text{NC}{{\text{H}}_{2}}\text{COOH} \right)$ and Amino caproic acid has the chemical formula $\left( {{\text{C}}_{6}}{{\text{H}}_{13}}{{\text{O}}_{2}}\text{N} \right)$. The reaction of both gives the polymer nylon 2-nylon 6 after removal of water molecules from the reactants.
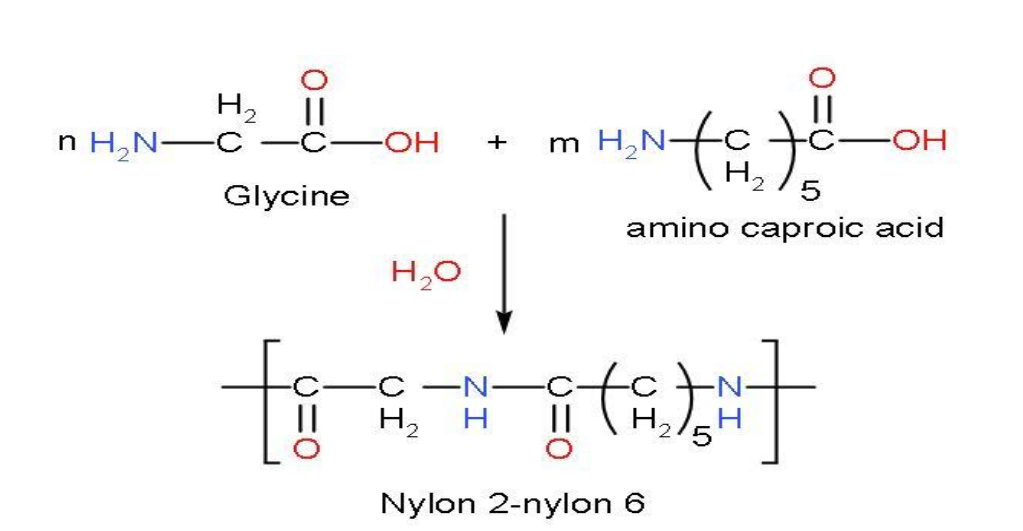
A condensation polymerization is in which monomers react with each other to form larger structural units while releasing water or methanol as by-product.
Biodegradable polymers are a type of polymer that breaks down by bacterial decomposition to result in natural by-products such as gases $\left( \text{C}{{\text{O}}_{2}},{{\text{N}}_{2}} \right)$, water and biomass.
The correct answer of this question is the monomers of biodegradable polymer, nylon 2-nylon 6 are glycine and amino caproic acid.
option ‘d’ is correct
Additional Information:
Applications of Nylon 2-nylon 6 are:
(1) It is used in synthesis of artificial fibers.
(2) Used to make strings of musical instruments and threads in bristles for toothbrushes.
Note:
Glycine is the only achiral amino acid. As glycine has two groups attached to it as the same which is two hydrogen atoms are attached to it. But the coming compounds of amino acids groups will have one hydrogen atom to be replaced with other groups. Like, Alanine has methyl group instead of hydrogen atom; $\text{C}{{\text{H}}_{3}}\text{CHN}{{\text{H}}_{2}}\text{COOH}$.
Complete step by step answer:
Polymerization is a process in which small molecules, known as monomers combine chemically to produce a very large network molecule which is called a polymer.
Monomers are the molecules that are bonded to other molecules to form a polymer.
There are two monomers of Nylon 2-nylon 6 named as Glycine $\left( \text{Gly} \right)$ with chemical formula $\left( {{\text{H}}_{2}}\text{NC}{{\text{H}}_{2}}\text{COOH} \right)$ and Amino caproic acid has the chemical formula $\left( {{\text{C}}_{6}}{{\text{H}}_{13}}{{\text{O}}_{2}}\text{N} \right)$. The reaction of both gives the polymer nylon 2-nylon 6 after removal of water molecules from the reactants.

A condensation polymerization is in which monomers react with each other to form larger structural units while releasing water or methanol as by-product.
Biodegradable polymers are a type of polymer that breaks down by bacterial decomposition to result in natural by-products such as gases $\left( \text{C}{{\text{O}}_{2}},{{\text{N}}_{2}} \right)$, water and biomass.
The correct answer of this question is the monomers of biodegradable polymer, nylon 2-nylon 6 are glycine and amino caproic acid.
option ‘d’ is correct
Additional Information:
Applications of Nylon 2-nylon 6 are:
(1) It is used in synthesis of artificial fibers.
(2) Used to make strings of musical instruments and threads in bristles for toothbrushes.
Note:
Glycine is the only achiral amino acid. As glycine has two groups attached to it as the same which is two hydrogen atoms are attached to it. But the coming compounds of amino acids groups will have one hydrogen atom to be replaced with other groups. Like, Alanine has methyl group instead of hydrogen atom; $\text{C}{{\text{H}}_{3}}\text{CHN}{{\text{H}}_{2}}\text{COOH}$.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




