
The number of stereocenters present in linear and cyclic structures of glucose are respectively:
(A) 4 and 5
(B) 5 and 5
(C) 4 and 4
(D) 5 and 4
Answer
233.1k+ views
Hint: Stereo Centre is nothing but a chiral center. A carbon which is attached to three or more different substituents is called a chiral center. If a molecule is optically active, that molecule should contain at least one chiral center in it.
Complete step by step answer:
* In the question it is given to find the number of stereocenters present in linear and cyclic structures of glucose.
* Sterocenter is nothing but the chiral center. If a carbon atom is attached to four different atoms are different groups then it is called stereocenter or chiral center.
* Chiral centers are optically active in nature.
* Linear structure and cyclic structures of glucose are as follows.
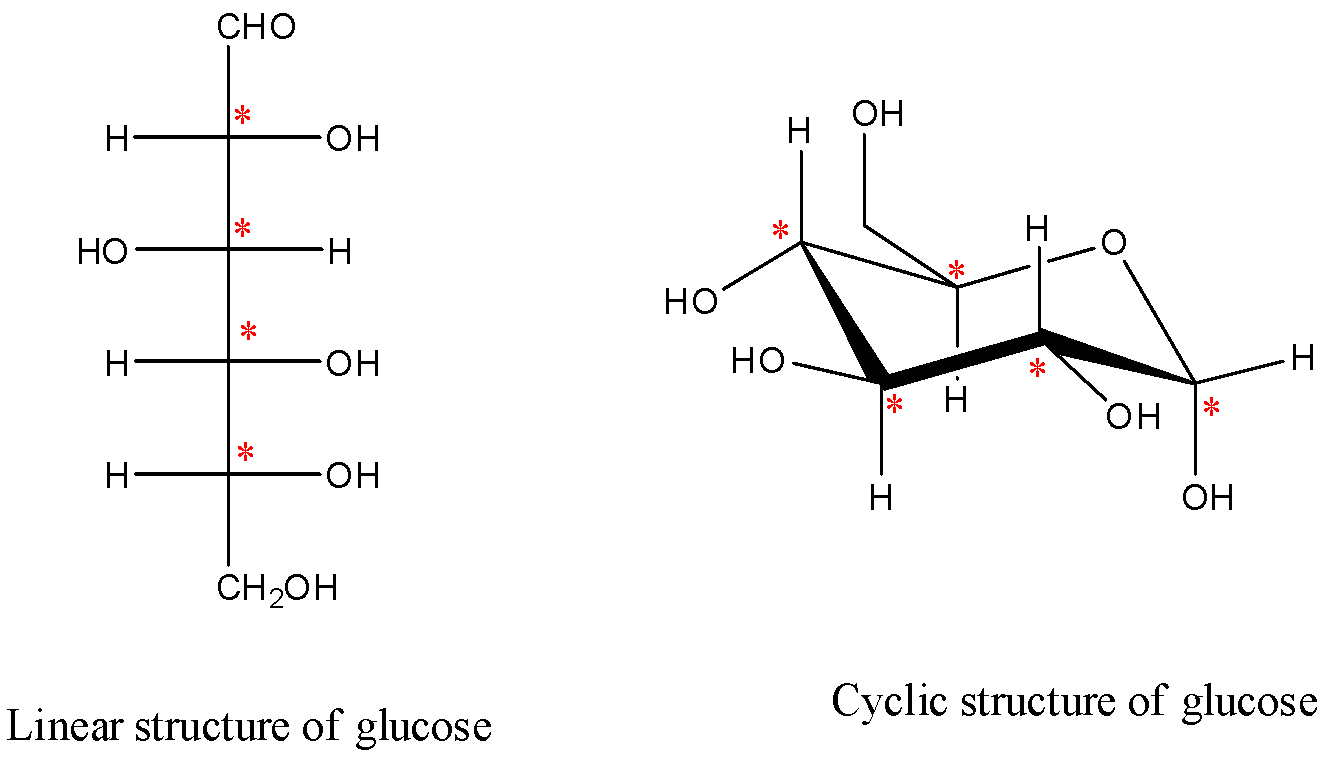
* The chiral centers are marked with red color stars.
* The number of chiral or stereo centers in linear structure of glucose are 4.
* The number of chiral or stereo centers in cyclic structure of glucose are 5.
* Therefore the number of chiral or stereo centers in linear structure and cyclic structures of glucose are 4. And 5 respectively.
So, the correct answer is A.
Additional information:
There is a reason behind one extra chiral carbon in cyclic glucose when compared to linear glucose is, after cyclisation of linear glucose one of the achiral carbon in linear structure is going to convert into chiral carbon.
Note: Linear structure of molecules is for our understanding purpose only, originally glucose exists in cyclic structures only with five chiral or stereo centers. The number of optically active isomers is also high for cyclic glucose structure when compared to linear structure of glucose.
Complete step by step answer:
* In the question it is given to find the number of stereocenters present in linear and cyclic structures of glucose.
* Sterocenter is nothing but the chiral center. If a carbon atom is attached to four different atoms are different groups then it is called stereocenter or chiral center.
* Chiral centers are optically active in nature.
* Linear structure and cyclic structures of glucose are as follows.

* The chiral centers are marked with red color stars.
* The number of chiral or stereo centers in linear structure of glucose are 4.
* The number of chiral or stereo centers in cyclic structure of glucose are 5.
* Therefore the number of chiral or stereo centers in linear structure and cyclic structures of glucose are 4. And 5 respectively.
So, the correct answer is A.
Additional information:
There is a reason behind one extra chiral carbon in cyclic glucose when compared to linear glucose is, after cyclisation of linear glucose one of the achiral carbon in linear structure is going to convert into chiral carbon.
Note: Linear structure of molecules is for our understanding purpose only, originally glucose exists in cyclic structures only with five chiral or stereo centers. The number of optically active isomers is also high for cyclic glucose structure when compared to linear structure of glucose.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




