
The reagent used in dehydrohalogenation is
A.\[{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\]
B.\[{{\text{K}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]
C. Alcoholic KOH
D. None of the above.
Answer
233.1k+ views
Hint: It occurs in a medium having a strong base. Thus when there is a strong base which favours elimination than substitution, then dehydrohalogenation can occur.
Complete step by step solution:
Let us try to understand Dehydrohalogenation first.
-Dehydrohalogenation is a reaction where a stronger base abstracts \[\text{ }\!\!\beta\!\!\text{ }\] hydrogen followed by eliminating halogens at adjacent carbon.
-Therefore, in this reaction strong base first abstracts the \[\text{ }\!\!\beta\!\!\text{ }\] hydrogen thereby collapsing the carbon \[\text{ }\!\!\beta\!\!\text{ }\]beta hydrogen bonds to form double bonds followed by breaking the carbon halogen bond thereby releasing the halide in that same medium. Therefore it generally proceeds through the E2 mechanism. (where the collapse of Beta hydrogen bond and leaving of halide occurs simultaneously.
Let us understand the concept of dehydrohalogenation through some examples.
-Dehydrohalogenation can be used to obtain alkene from Alkyl halides-
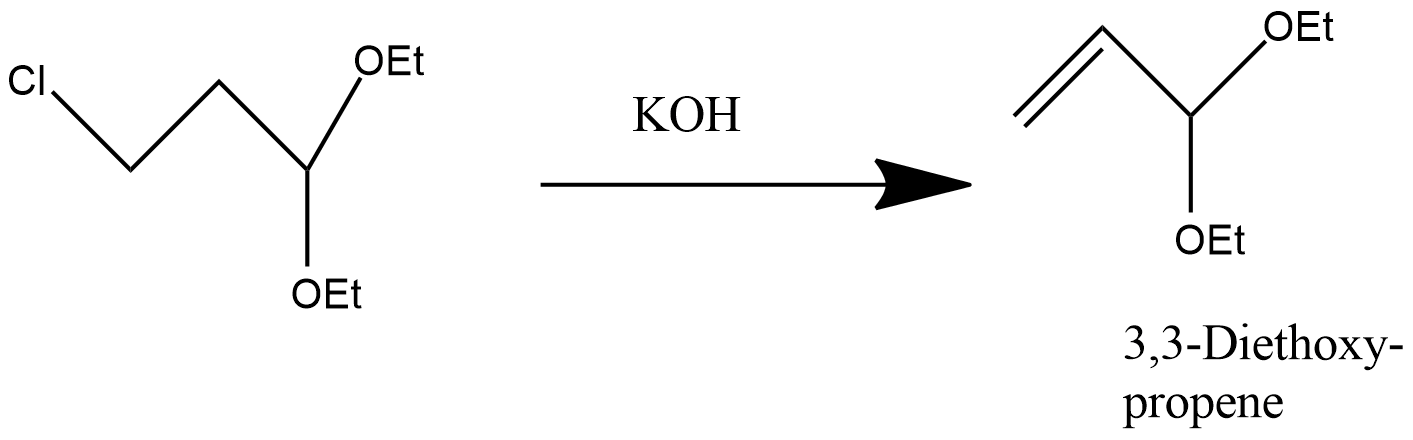
-Dehydrohalogenation can be used to obtain Diene from Alkyl halides-
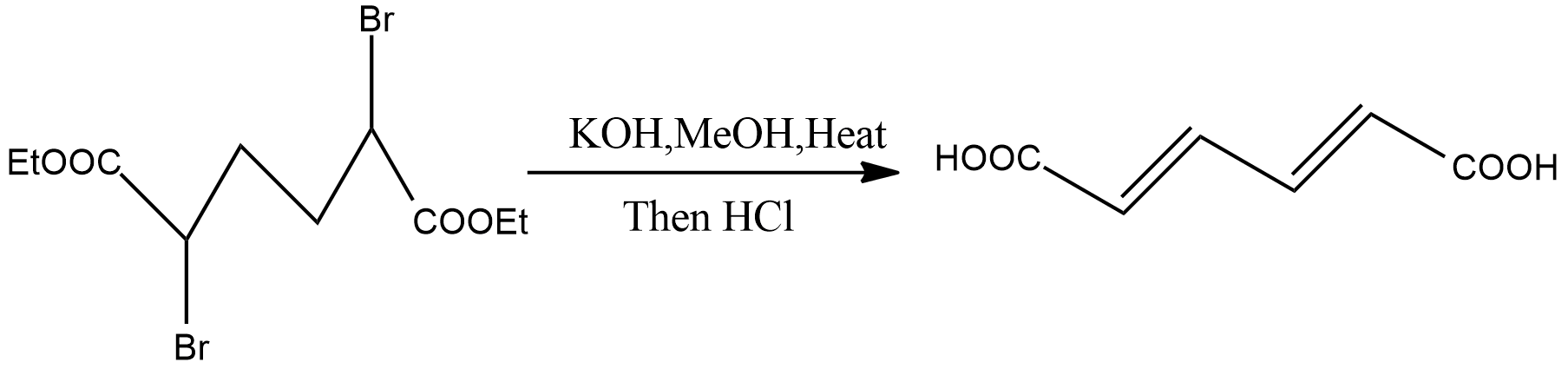
-Dehydration of Vic-dihalides, Gem-dihalides to form alkynes:
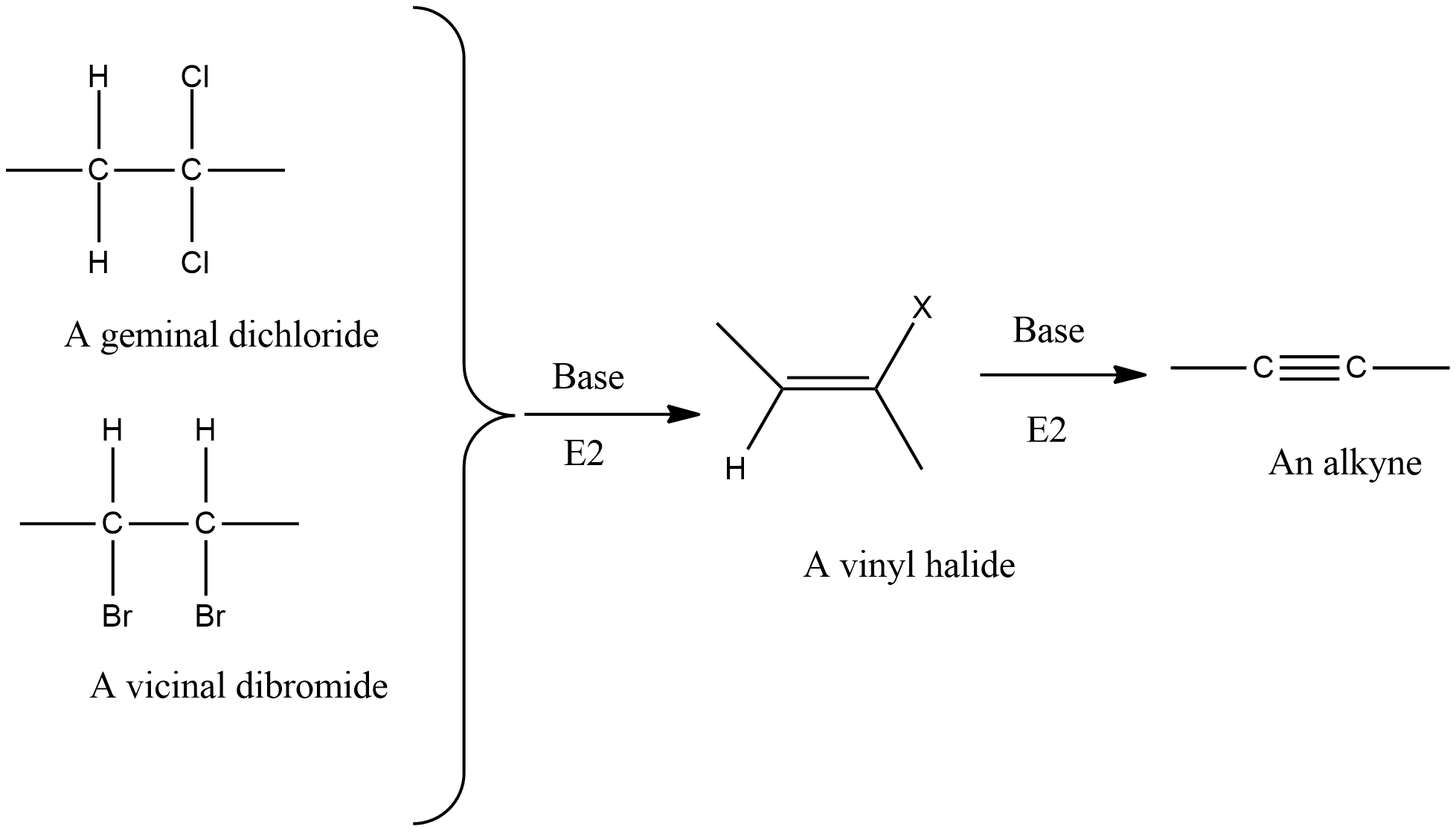
Here, the second step is slower as vinyl halide is resonance stabilized therefore highest temperature and stronger bases are required.
When we use alcoholic KOH is present as a reagent, the negative part of the reagent, that is $\text{O}{{\text{H}}^{-}}$it acts as a base and abstracts the $\text{ }\!\!\beta\!\!\text{ }$ hydrogen from the saturated substrate ( alkyl halide) present and transforms it to an alkene in the product, thereby undergoing elimination reaction.
Therefore, Option C is the correct option.
Additional information: Sodium amide, $NaN{{H}_{2}}$, in liquid ammonia as a solvent was the preferred base for many years. In some cases, $NaN{{H}_{2}}$ is used in mineral oil. Now amide ions such as lithium diisopropylamide, LDA, is routinely used. A hot, alcoholic KOH solution or an alkoxide ion, especially potassium t-butyl alcohol, DMSO, or in THF is also commonly used to effect elimination of HX from vinyl halide. The reaction can be carried out stepwise, via the formation of a vinyl halide, or in one step, generating the alkyne directly. Thus the addition of 1,2-dibromohexane to sodium amide in liquid ammonia followed by evaporation of the solvent and aqueous workup gives 1-hexyne.
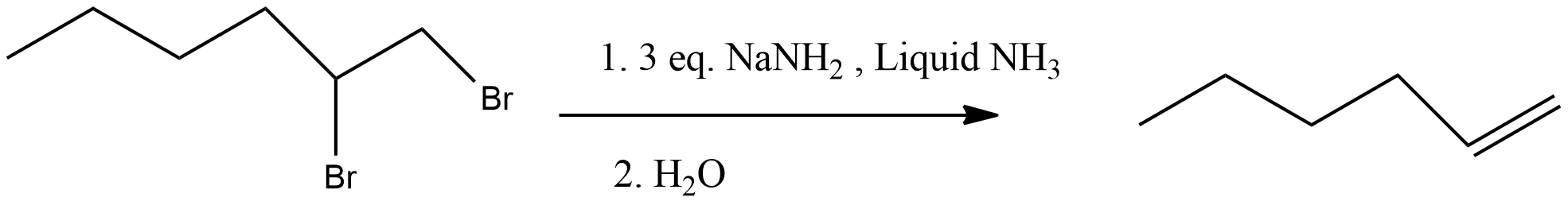
Three equivalents of $NaN{{H}_{2}}$ is necessary for the preparation of terminal alkyne because as the alkyne forms, its acidic terminal hydrogen immediately protonates an equivalent amount of base. Because vicinal dihalides are readily available from alkenes through halogen addition and geminal dichlorides are easily available by the treatment of $PC{{l}_{5}}$ on aldehyde or ketone the above double dehydrogenation is a unique technique to obtain alkyne.
Note: Since elimination and substitution can both occur in the same medium therefore for dehydrohalogenation we need to provide better reagent and reaction condition for elimination to undergo dehydrohalogenation quite easily. Therefore we have to use strong bases like alcoholic KOH,$O{{R}^{-}}$,$NH_{2}^{-}$ etc.
Complete step by step solution:
Let us try to understand Dehydrohalogenation first.
-Dehydrohalogenation is a reaction where a stronger base abstracts \[\text{ }\!\!\beta\!\!\text{ }\] hydrogen followed by eliminating halogens at adjacent carbon.
-Therefore, in this reaction strong base first abstracts the \[\text{ }\!\!\beta\!\!\text{ }\] hydrogen thereby collapsing the carbon \[\text{ }\!\!\beta\!\!\text{ }\]beta hydrogen bonds to form double bonds followed by breaking the carbon halogen bond thereby releasing the halide in that same medium. Therefore it generally proceeds through the E2 mechanism. (where the collapse of Beta hydrogen bond and leaving of halide occurs simultaneously.
Let us understand the concept of dehydrohalogenation through some examples.
-Dehydrohalogenation can be used to obtain alkene from Alkyl halides-

-Dehydrohalogenation can be used to obtain Diene from Alkyl halides-

-Dehydration of Vic-dihalides, Gem-dihalides to form alkynes:

Here, the second step is slower as vinyl halide is resonance stabilized therefore highest temperature and stronger bases are required.
When we use alcoholic KOH is present as a reagent, the negative part of the reagent, that is $\text{O}{{\text{H}}^{-}}$it acts as a base and abstracts the $\text{ }\!\!\beta\!\!\text{ }$ hydrogen from the saturated substrate ( alkyl halide) present and transforms it to an alkene in the product, thereby undergoing elimination reaction.
Therefore, Option C is the correct option.
Additional information: Sodium amide, $NaN{{H}_{2}}$, in liquid ammonia as a solvent was the preferred base for many years. In some cases, $NaN{{H}_{2}}$ is used in mineral oil. Now amide ions such as lithium diisopropylamide, LDA, is routinely used. A hot, alcoholic KOH solution or an alkoxide ion, especially potassium t-butyl alcohol, DMSO, or in THF is also commonly used to effect elimination of HX from vinyl halide. The reaction can be carried out stepwise, via the formation of a vinyl halide, or in one step, generating the alkyne directly. Thus the addition of 1,2-dibromohexane to sodium amide in liquid ammonia followed by evaporation of the solvent and aqueous workup gives 1-hexyne.
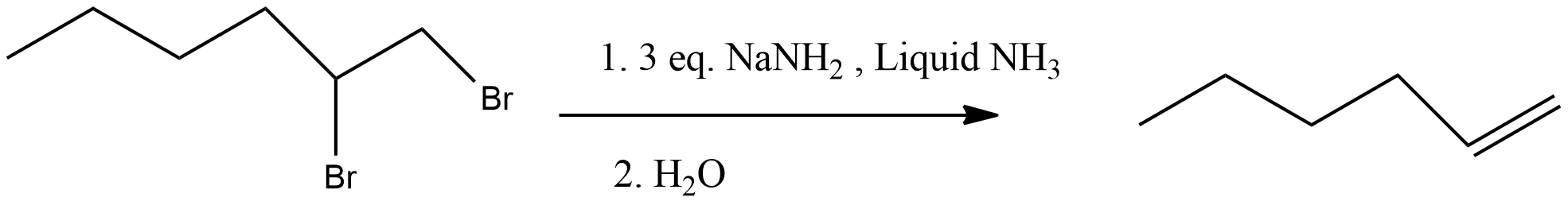
Three equivalents of $NaN{{H}_{2}}$ is necessary for the preparation of terminal alkyne because as the alkyne forms, its acidic terminal hydrogen immediately protonates an equivalent amount of base. Because vicinal dihalides are readily available from alkenes through halogen addition and geminal dichlorides are easily available by the treatment of $PC{{l}_{5}}$ on aldehyde or ketone the above double dehydrogenation is a unique technique to obtain alkyne.
Note: Since elimination and substitution can both occur in the same medium therefore for dehydrohalogenation we need to provide better reagent and reaction condition for elimination to undergo dehydrohalogenation quite easily. Therefore we have to use strong bases like alcoholic KOH,$O{{R}^{-}}$,$NH_{2}^{-}$ etc.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




