
Which of the following exhibits linkage isomerism?
(A)- \[[Co{{(N{{H}_{3}})}_{5}}S{{O}_{4}}]Br\]
(B)- \[[Co{{({{H}_{2}}O)}_{6}}]C{{l}_{3}}\]
(C)- \[[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]C{{l}_{2}}\]
(D)- \[[Co{{(N{{H}_{3}})}_{6}}][Cr{{(CN)}_{6}}]\]
Answer
233.1k+ views
Hint: The coordination complexes that show linkage isomerism must have one special type of ligand which has two donor atoms, but only one of the donor atoms can bind to the central metal atom at a time.
Complete step by step solution:
- The coordination complexes that show linkage isomerism have ambidentate ligand.
- Ambidentate ligands are those which have two donor atoms, but only one of the donor atoms can bind to the central metal atom at a time.
- ‘Ambi’ means both and dentate means the denticity of the ligand.
- Denticity is the number of the donor atoms of the ligand that binds to the central metal atom in the coordination complex.
- Here, \[[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]C{{l}_{2}}\]exhibits linkage isomerism.
\[N{{O}_{2}}\] is the ambidentate ligand in this coordination complex. This ligand is the reason the coordination complex shows the linkage isomerism.
When the central metal atom is bonded with nitrogen, the ligand is\[N{{O}_{2}}\]. When the metal atom is bonded with oxygen, the ligand is\[ONO\].
- Other examples of the ambidentate ligand are \[C{{N}^{-}}\]and\[N{{C}^{-}}\], \[SC{{N}^{-}}\]and\[NC{{S}^{-}}\].
- \[[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]C{{l}_{2}}\]and \[[Co{{(N{{H}_{3}})}_{5}}(ONO)]C{{l}_{2}}\]are the linkage isomers.
So, option C is the correct option.
Additional Information:
Ligands can be monodentate, bidentate, and multidentate as well depending upon the number of the donor atoms of the ligand binding to the central metal atom.
Note \[N{{O}_{2}}\]is called a nitro ligand. When the donation is from the nitrogen to the metal center, it is nitro complex. The linkage of the ligand is as follow
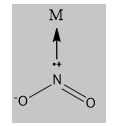
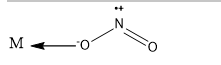
\[ONO\]is called nitrito ligand. When the donation is from the nitrogen to the metal centre, it is nitrito complex. The linkage is as follows:
The complexes are octahedral in shape.
Complete step by step solution:
- The coordination complexes that show linkage isomerism have ambidentate ligand.
- Ambidentate ligands are those which have two donor atoms, but only one of the donor atoms can bind to the central metal atom at a time.
- ‘Ambi’ means both and dentate means the denticity of the ligand.
- Denticity is the number of the donor atoms of the ligand that binds to the central metal atom in the coordination complex.
- Here, \[[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]C{{l}_{2}}\]exhibits linkage isomerism.
\[N{{O}_{2}}\] is the ambidentate ligand in this coordination complex. This ligand is the reason the coordination complex shows the linkage isomerism.
When the central metal atom is bonded with nitrogen, the ligand is\[N{{O}_{2}}\]. When the metal atom is bonded with oxygen, the ligand is\[ONO\].
- Other examples of the ambidentate ligand are \[C{{N}^{-}}\]and\[N{{C}^{-}}\], \[SC{{N}^{-}}\]and\[NC{{S}^{-}}\].
- \[[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]C{{l}_{2}}\]and \[[Co{{(N{{H}_{3}})}_{5}}(ONO)]C{{l}_{2}}\]are the linkage isomers.
So, option C is the correct option.
Additional Information:
Ligands can be monodentate, bidentate, and multidentate as well depending upon the number of the donor atoms of the ligand binding to the central metal atom.
Note \[N{{O}_{2}}\]is called a nitro ligand. When the donation is from the nitrogen to the metal center, it is nitro complex. The linkage of the ligand is as follow

\[ONO\]is called nitrito ligand. When the donation is from the nitrogen to the metal centre, it is nitrito complex. The linkage is as follows:

The complexes are octahedral in shape.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

Area of an Octagon Formula Explained Simply

Absolute Pressure Formula Explained: Key Equation & Examples

Central Angle of a Circle Formula Explained Quickly

Difference Between Vapor and Gas: JEE Main 2026

Difference Between Atom and Molecule: JEE Main 2026

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Jan 21 Shift 1 Question Papers with Solutions & Answer Keys – Detailed Day 1 Analysis

JEE Main Response Sheet 2026 Released – Key Dates and Official Updates by NTA

JEE Main 2026 Answer Key OUT – Download Session 1 PDF, Response Sheet & Challenge Link

JEE Main Marks vs Percentile 2026: Calculate Percentile and Rank Using Marks

JEE Main 2026 Jan 22 Shift 1 Today Paper Live Analysis With Detailed Solutions

Other Pages
Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages

One Day International Cricket

Valentine Week 2026: Complete List of Valentine Week Days & Meaning of Each Day

List of Highest T20 Scores in International Cricket

Makar Sankranti Wishes: Happy Makar Sankranti Wishes in Marathi, Hindi, Kannada, and English

What is the Full Form of UGC? Detailed Guide for Students




