
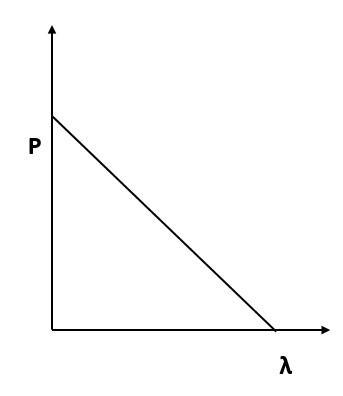
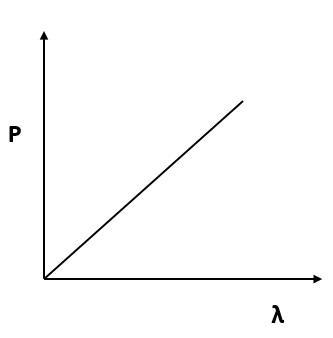
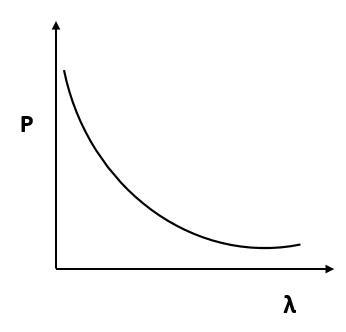
Which of the following figures represent the variation of particle momentum and the associated de-Broglie wavelength?
(A)
(B)
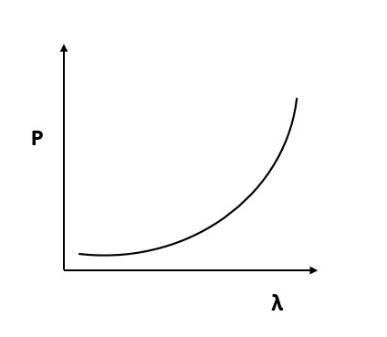
(C)
(D)




Answer
232.8k+ views
Hint: The relation between de Broglie wavelength and linear momentum is calculated from the energy of the photon. De Broglie’s equation equates de Broglie wavelength to the ratio of Planck’s constant and linear momentum.
Formula Used: The formulae used in the solution are given here.
Momentum of a photon is given by-
$P = \dfrac{E}{c} = \dfrac{h}{\lambda }$ where $E$ is the energy of the photon, $c$ is the speed of light in vacuum, $h$ is the Planck’s constant and $\lambda $ is the de Broglie wavelength.
Complete Step by Step Solution: The wavelength that is associated with an object in relation to its momentum and mass is known as de Broglie wavelength. A particle’s de Broglie wavelength is usually inversely proportional to its force.
Momentum of a photon is given by-
$P = \dfrac{E}{c} = \dfrac{h}{\lambda }$ where $E$ is the energy of the photon, $c$ is the speed of light in vacuum, $h$ is the Planck’s constant and $\lambda $ is the de Broglie wavelength.
According to de Broglie, $p = \dfrac{h}{\lambda }$ or $p\alpha \dfrac{1}{\lambda }$.
By this relation we can conclude that the linear momentum of a photon is inversely proportional to the de Broglie wavelength. The graph of $p$ vs $\lambda$ shall be a rectangular hyperbola.
It will look like,
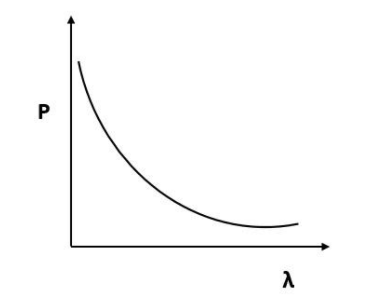
Hence, the correct answer is Option D.
Note: It is said that matter has a dual nature of wave-particles. De Broglie waves named after the discoverer Louis de Broglie, is the property of a material object that varies in time or space while behaving similar to waves. It is also called matter-waves. It holds great similarity to the dual nature of light which behaves as particle and wave, which has been proven experimentally.
The physicist Louis de Broglie suggested that particles might have both wave properties and particle properties. The wave nature of electrons was also detected experimentally to substantiate the suggestion of Louis de Broglie.
The objects which we see in day-to-day life have wavelengths which are very small and invisible, hence, we do not experience them as waves. However, de Broglie wavelengths are quite visible in the case of subatomic particles.
Formula Used: The formulae used in the solution are given here.
Momentum of a photon is given by-
$P = \dfrac{E}{c} = \dfrac{h}{\lambda }$ where $E$ is the energy of the photon, $c$ is the speed of light in vacuum, $h$ is the Planck’s constant and $\lambda $ is the de Broglie wavelength.
Complete Step by Step Solution: The wavelength that is associated with an object in relation to its momentum and mass is known as de Broglie wavelength. A particle’s de Broglie wavelength is usually inversely proportional to its force.
Momentum of a photon is given by-
$P = \dfrac{E}{c} = \dfrac{h}{\lambda }$ where $E$ is the energy of the photon, $c$ is the speed of light in vacuum, $h$ is the Planck’s constant and $\lambda $ is the de Broglie wavelength.
According to de Broglie, $p = \dfrac{h}{\lambda }$ or $p\alpha \dfrac{1}{\lambda }$.
By this relation we can conclude that the linear momentum of a photon is inversely proportional to the de Broglie wavelength. The graph of $p$ vs $\lambda$ shall be a rectangular hyperbola.
It will look like,

Hence, the correct answer is Option D.
Note: It is said that matter has a dual nature of wave-particles. De Broglie waves named after the discoverer Louis de Broglie, is the property of a material object that varies in time or space while behaving similar to waves. It is also called matter-waves. It holds great similarity to the dual nature of light which behaves as particle and wave, which has been proven experimentally.
The physicist Louis de Broglie suggested that particles might have both wave properties and particle properties. The wave nature of electrons was also detected experimentally to substantiate the suggestion of Louis de Broglie.
The objects which we see in day-to-day life have wavelengths which are very small and invisible, hence, we do not experience them as waves. However, de Broglie wavelengths are quite visible in the case of subatomic particles.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students

Understanding Electromagnetic Waves and Their Importance




