
Which of the following is least basic?
(A)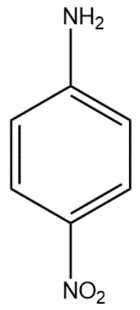
(B) 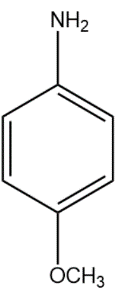
(C) 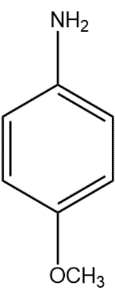
(D) 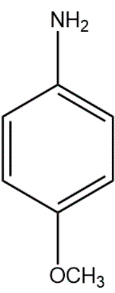
Answer
233.1k+ views
Hint: The four organic compounds have the amine group in common. The basic strength of the compounds can be determined by the stability of the conjugate acid formed. Only when the conjugate acid formed is stable, the lone pair will accept the incoming proton. The groups attached will contribute to either stabilizing or destabilizing the positive charge after protonation.
Complete step by step solution:
Electronic factors are the factor that influences various organic reactions and rearrangements. Electronic effects are significantly observed in organic aromatic compounds.
Electronic factors are:
-Inductive effect
-Resonance
-Mesomeric effect
-Electromeric effect
-Hyperconjugation.
We will now identify at which position of the aromatic ring, the following effects are observed.
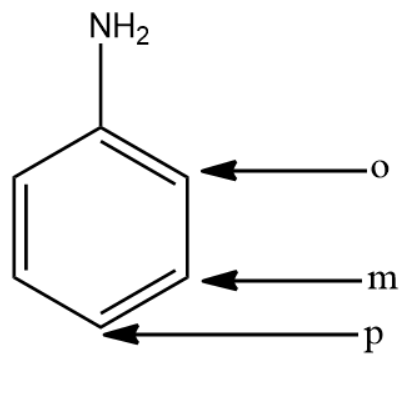
In the above compound,
-o stands for ortho position
-m stands for meta-position
-p stands for para position
In compound (A), the nitro group attached to the para position withdraws electrons from the aromatic system, thus making the protonated amine group unstable by resonance.
In compound (B), the methoxy group attached to the para position donates electrons from oxygen atom to the aromatic system, stabilising the positive charge on the conjugate acid.
In compound (C), the chlorine atom attached to the para position acts as a mild deactivating agent using the inductive effect.
In compound (D), the methyl group attached to the para position stabilises the protonated amine by inductive and hyperconjugation effect.
Compound (A) is least basic because as we discussed before, resonance has greater priority than the inductive effect in stabilising as well as in destabilising the aromatic system.
Therefore, the correct answer is option (A).
Note: Resonance effect is mainly observed in the ortho and para position of the aromatic ring and the only significant electronic effect observed at the meta position is the inductive effect. Resonance has much more priority than inductive effect while determining the acidic strength of phenols or carboxylic acids. This is because resonance involves the actual displacement of electrons, unlike partial positive/negative charge which is observed in the inductive effect.
Complete step by step solution:
Electronic factors are the factor that influences various organic reactions and rearrangements. Electronic effects are significantly observed in organic aromatic compounds.
Electronic factors are:
-Inductive effect
-Resonance
-Mesomeric effect
-Electromeric effect
-Hyperconjugation.
We will now identify at which position of the aromatic ring, the following effects are observed.

In the above compound,
-o stands for ortho position
-m stands for meta-position
-p stands for para position
In compound (A), the nitro group attached to the para position withdraws electrons from the aromatic system, thus making the protonated amine group unstable by resonance.
In compound (B), the methoxy group attached to the para position donates electrons from oxygen atom to the aromatic system, stabilising the positive charge on the conjugate acid.
In compound (C), the chlorine atom attached to the para position acts as a mild deactivating agent using the inductive effect.
In compound (D), the methyl group attached to the para position stabilises the protonated amine by inductive and hyperconjugation effect.
Compound (A) is least basic because as we discussed before, resonance has greater priority than the inductive effect in stabilising as well as in destabilising the aromatic system.
Therefore, the correct answer is option (A).
Note: Resonance effect is mainly observed in the ortho and para position of the aromatic ring and the only significant electronic effect observed at the meta position is the inductive effect. Resonance has much more priority than inductive effect while determining the acidic strength of phenols or carboxylic acids. This is because resonance involves the actual displacement of electrons, unlike partial positive/negative charge which is observed in the inductive effect.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




