
Which of the following is the most reactive in Friedel craft acylation reaction?
(A) 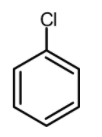
(B) 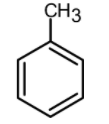
(C) 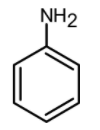
(D) 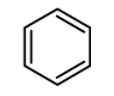
Answer
233.1k+ views
Hint: The Friedel craft reactions are electrophilic substitution reactions. The activation or deactivation of the benzene ring towards the electrophilic substitution reaction depends on the substituents. The electron-donating group activates the ring towards the electrophilic substitution reaction and the electron-withdrawing groups like $\text{-Cl}$ or nitro deactivates the ring. The amine reacts differently in Friedel's craft reaction. It forms the complex and does not give the acylated product.
Complete step by step solution:
-The Friedel-Crafts acylation reaction is an electrophilic substitution reaction. It is used for the synthesis of monoacetylated products.it is a reaction between arene and the acyl chloride $\text{(R-COCl)}$ to give the acylated products.
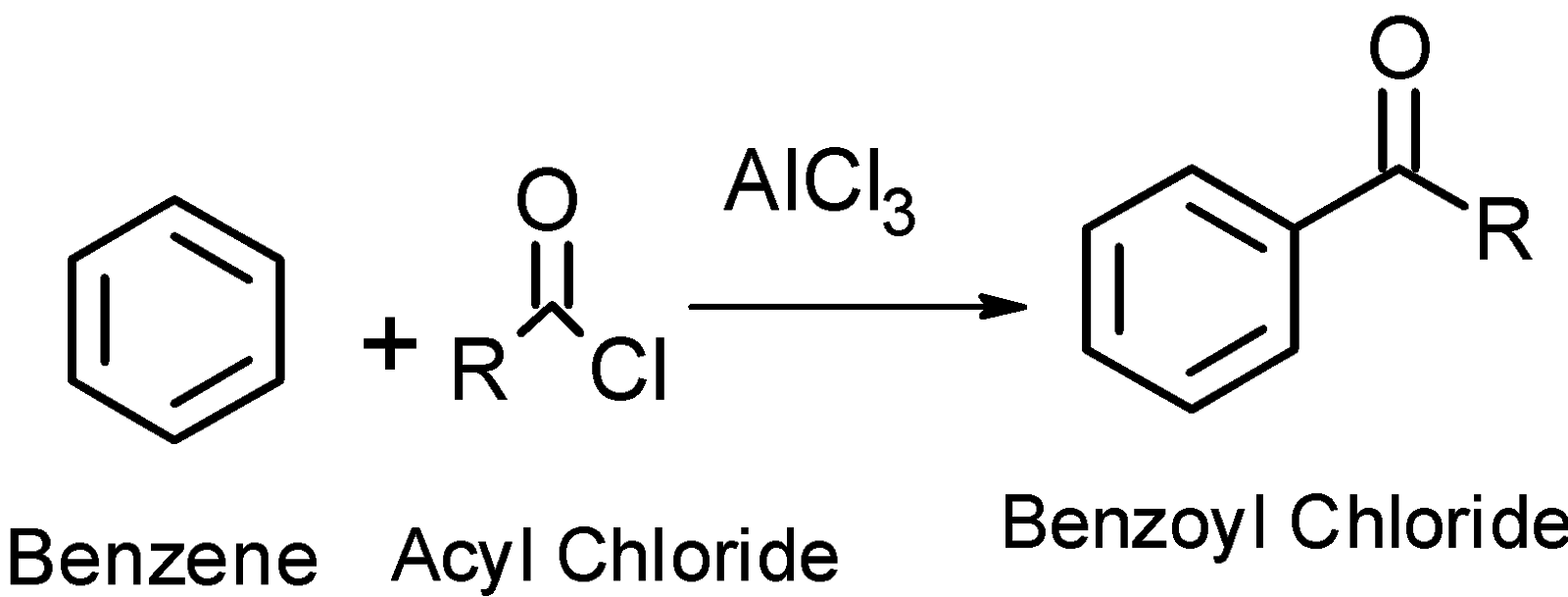
-The Friedel Craft reaction is electrophilic substitution reactions.in which the electrophile displaces the functional group existing on the ring.
In the Friedel crafts, the acylation reaction \[\text{AlC}{{\text{l}}_{\text{3}}}\]acts as a catalyst that further reacts with the acyl group to convert it into the electrophile.
-This electrophile attacks on the electron-rich positions. This is para and Meta positions in the ring. This results in the formation of the product.
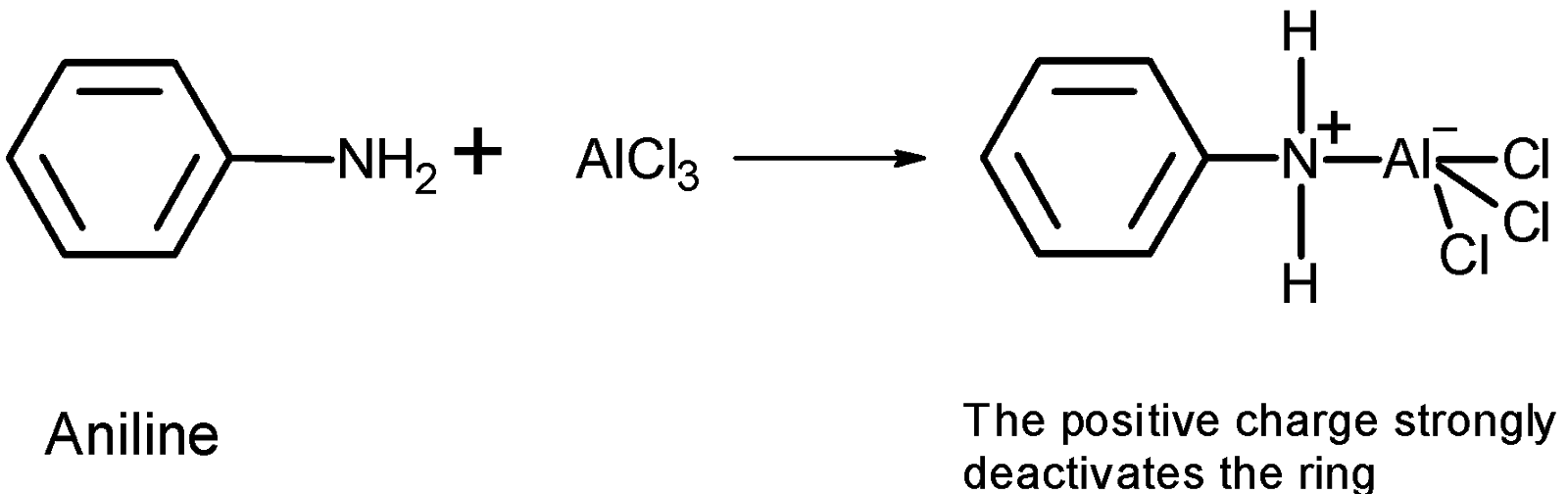
-Friedel-Crafts reactions cannot be performed for the aromatic system which contains a \[\text{N}{{\text{H}}_{\text{2}}}\text{, NHR, or N}{{\text{R}}_{\text{2}}}\text{ }\!\!~\!\!\text{ }\] substituent. Such as aniline, etc. This is because the lone pair on the amines makes it Lewis base and thus the lone pair of electrons react with the Lewis acid which is $\text{AlC}{{\text{l}}_{\text{3}}}$.
The reaction generates a positive charge at the next to the ring results in the deactivation of the ring. Thus the Friedel craft acylation does not occur.
Therefore from the given compounds, the aniline does not undergo the Friedel crafts acylation. $\text{AlC}{{\text{l}}_{\text{3}}}$ reacts with the amino group $\text{(-N}{{\text{H}}_{\text{2}}}\text{)}$ and forms an insoluble complex.
Now let us have a look at chlorobenzene, benzene, and the toluene. The order of reactivity of the rings towards the electrophilic substitution is found to be greater for toluene than benzene than the chlorobenzene. The ortho and para positions are reactive towards the electrophile. The reaction takes place even in presence of poor electrophile. Hence the methyl group is 25 times more reactive than the benzene.
\[\begin{array}{*{35}{l}}
\text{Toluene}\rangle \text{Benzene}\rangle \text{Chlorobenzene} \\
\text{ }\!\!~\!\!\text{ } \\
\end{array}\]
Therefore, the group which is highly reactive towards Friedel craft acylation is the toluene.
Hence, (B) is the correct option.
Note: $\text{AlC}{{\text{l}}_{\text{3}}}$ acts as a catalyst. In such a question remember the electron-donating and electron-withdrawing group. Friedel-Crafts fails when we use the compound with a nitro group such as nitrobenzene. Unlike polyalkylation in Friedel craft alkylation, the acylation reaction does not for poly acylated products.
Complete step by step solution:
-The Friedel-Crafts acylation reaction is an electrophilic substitution reaction. It is used for the synthesis of monoacetylated products.it is a reaction between arene and the acyl chloride $\text{(R-COCl)}$ to give the acylated products.

-The Friedel Craft reaction is electrophilic substitution reactions.in which the electrophile displaces the functional group existing on the ring.
In the Friedel crafts, the acylation reaction \[\text{AlC}{{\text{l}}_{\text{3}}}\]acts as a catalyst that further reacts with the acyl group to convert it into the electrophile.
-This electrophile attacks on the electron-rich positions. This is para and Meta positions in the ring. This results in the formation of the product.
-Friedel-Crafts reactions cannot be performed for the aromatic system which contains a \[\text{N}{{\text{H}}_{\text{2}}}\text{, NHR, or N}{{\text{R}}_{\text{2}}}\text{ }\!\!~\!\!\text{ }\] substituent. Such as aniline, etc. This is because the lone pair on the amines makes it Lewis base and thus the lone pair of electrons react with the Lewis acid which is $\text{AlC}{{\text{l}}_{\text{3}}}$.
The reaction generates a positive charge at the next to the ring results in the deactivation of the ring. Thus the Friedel craft acylation does not occur.
Therefore from the given compounds, the aniline does not undergo the Friedel crafts acylation. $\text{AlC}{{\text{l}}_{\text{3}}}$ reacts with the amino group $\text{(-N}{{\text{H}}_{\text{2}}}\text{)}$ and forms an insoluble complex.

Now let us have a look at chlorobenzene, benzene, and the toluene. The order of reactivity of the rings towards the electrophilic substitution is found to be greater for toluene than benzene than the chlorobenzene. The ortho and para positions are reactive towards the electrophile. The reaction takes place even in presence of poor electrophile. Hence the methyl group is 25 times more reactive than the benzene.
\[\begin{array}{*{35}{l}}
\text{Toluene}\rangle \text{Benzene}\rangle \text{Chlorobenzene} \\
\text{ }\!\!~\!\!\text{ } \\
\end{array}\]
Therefore, the group which is highly reactive towards Friedel craft acylation is the toluene.
Hence, (B) is the correct option.
Note: $\text{AlC}{{\text{l}}_{\text{3}}}$ acts as a catalyst. In such a question remember the electron-donating and electron-withdrawing group. Friedel-Crafts fails when we use the compound with a nitro group such as nitrobenzene. Unlike polyalkylation in Friedel craft alkylation, the acylation reaction does not for poly acylated products.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




