
Which of the following polymers is used in paints?
(a) Gutta percha
(b) Melamine
(c) Buna-S
(d) Novolac
Answer
233.1k+ views
Hint: Polymers are macromolecules. The polymer that is used in paints is prepared through condensation polymerisation between phenol and methanal; and it is an amorphous thermoplastic.
Complete step by step answer:
Before diving into the question, we need to understand polymers. Polymers are large molecules that are composed of smaller repeating units called monomers that are chemically linked to each other. They can be single chained, they can also be branched or they can be cross-linked. Generally in synthetic polymers, the links in the chain are the same as that of its neighbours while that is not the case in many natural polymers such as proteins.
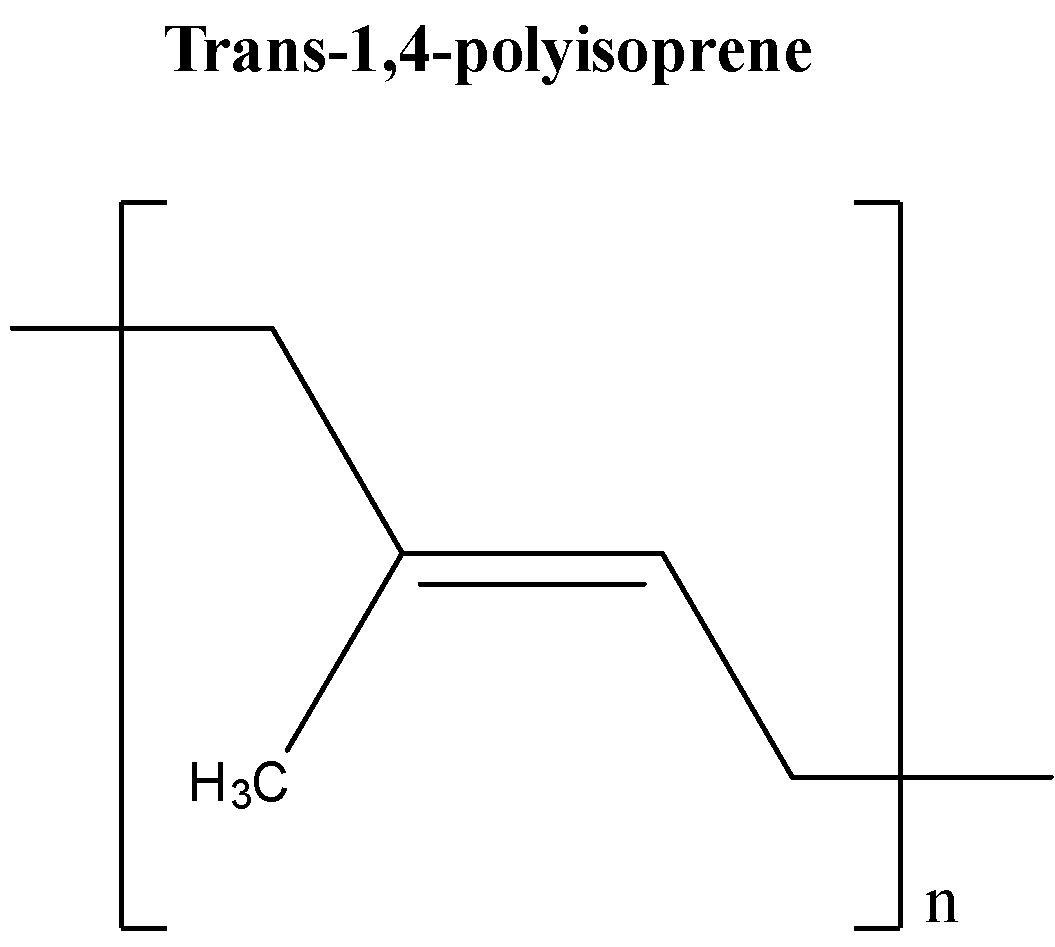
Gutta-percha is a polymer of isoprene (trans-1, 4-polyisoprene). It is non-conductive in nature and is rubber like elastomer. It is also naturally inert. Its chemical structure is given below:
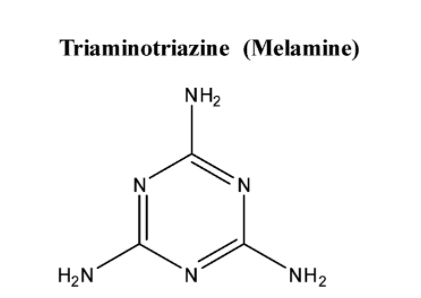
Melamine is not a polymer but a heterocyclic organic compound which is a colourless crystalline solid. Its chemical structure is given below:
Melamine is used as a starting material for the manufacture of melamine-formaldehyde polymer known as melmac through condensation polymerization between melamine and formaldehyde. Many non-breakable plastic crockery, cups and plates are made of melmac.
Buna-S is prepared by the addition polymerization of 1,3-butadiene and styrene. The reaction is given below:

Buna-S has many applications, for example it is used in rubber cutting boards, sealing agents in building application etc.
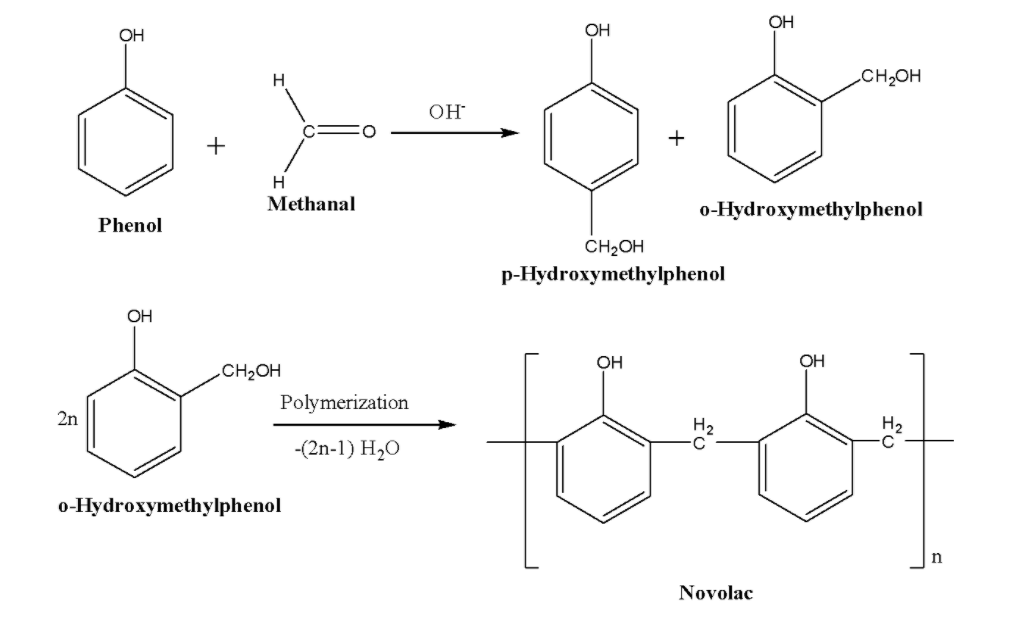
Novolac is prepared by condensation of phenol with methanal using an acid or a base as a catalyst. The reaction gives the products: o-Hydroxymethylphenol and p-Hydroxymethylphenol. These derivatives further react with phenol to form a linear polymer novolac. Novolac is widely used in paints. The reaction is given below:
Hence the correct answer is (d) Novolac.
Note:
The chemical properties of the members of a homologous series similar though the first member may vary considerably from the rest of the members. The successive members of a homologous series differ by a \[C{{H}_{2}}\] group or by 14 mass units.
Complete step by step answer:
Before diving into the question, we need to understand polymers. Polymers are large molecules that are composed of smaller repeating units called monomers that are chemically linked to each other. They can be single chained, they can also be branched or they can be cross-linked. Generally in synthetic polymers, the links in the chain are the same as that of its neighbours while that is not the case in many natural polymers such as proteins.
Gutta-percha is a polymer of isoprene (trans-1, 4-polyisoprene). It is non-conductive in nature and is rubber like elastomer. It is also naturally inert. Its chemical structure is given below:

Melamine is not a polymer but a heterocyclic organic compound which is a colourless crystalline solid. Its chemical structure is given below:

Melamine is used as a starting material for the manufacture of melamine-formaldehyde polymer known as melmac through condensation polymerization between melamine and formaldehyde. Many non-breakable plastic crockery, cups and plates are made of melmac.
Buna-S is prepared by the addition polymerization of 1,3-butadiene and styrene. The reaction is given below:

Buna-S has many applications, for example it is used in rubber cutting boards, sealing agents in building application etc.
Novolac is prepared by condensation of phenol with methanal using an acid or a base as a catalyst. The reaction gives the products: o-Hydroxymethylphenol and p-Hydroxymethylphenol. These derivatives further react with phenol to form a linear polymer novolac. Novolac is widely used in paints. The reaction is given below:

Hence the correct answer is (d) Novolac.
Note:
The chemical properties of the members of a homologous series similar though the first member may vary considerably from the rest of the members. The successive members of a homologous series differ by a \[C{{H}_{2}}\] group or by 14 mass units.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




