
White phosphorus ${P_4}$ has the following characteristics.
(This question has multiple correct options)
(A) 6 P-P single bonds
(B) 4 P-P single bonds
(C) 4 lone pair of electrons
(D) P-P-P angle of ${60^\circ }$
Answer
232.8k+ views
Hint: Phosphorus is 15 group elements. Phosphorus is an essential constituent of animal and plant matter. It is present in bones as well as in living cells.
Complete step by step answer:
Phosphorus exists in several allotropic forms.
Example: White phosphorus, red phosphorus, black phosphorus, etc.
White phosphorus is in gaseous state and is a waxy solid consisting of reactive ${p_4}$tetrahedron.

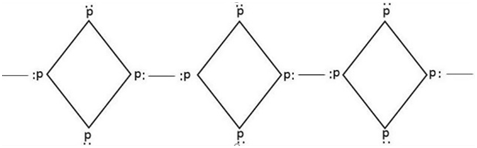
The structure of phosphorus shows 6 covalent bonds between P-atom and 4 lone pairs of electrons.
Therefore, from the above explanation, the correct option is [A] 6 p-p single bond and [C] 4 lone pair of electrons
Additional information:
White phosphorus is very reactive because ${p_4}$ rings are held together by physical bonding but no chemical bonding.
These ${p_4}$ rings are in unstable arrangement.
The red phosphorus consists of complex chain structure formed by opening of ${p_4}$cage.
Black phosphorus has a layered structure and most thermodynamically stable form.
Phosphorus can be considered as light solid.
White phosphorus is very reactive and spontaneously ignites at ${30^\circ }C$ in moist air so usually it is stored under water. So that exposure to air can be prevented.
Red phosphorus is stable at room temperature but can be converted to white phosphorus by heat, sunlight or friction.
Note: White phosphorus crystallizes in a cubic system. Phosphorus atomic size is more so it can form a single bond with other phosphorus atoms. Hence it exists as tetra atomic molecules.
Complete step by step answer:
Phosphorus exists in several allotropic forms.
Example: White phosphorus, red phosphorus, black phosphorus, etc.
White phosphorus is in gaseous state and is a waxy solid consisting of reactive ${p_4}$tetrahedron.

The structure of phosphorus shows 6 covalent bonds between P-atom and 4 lone pairs of electrons.
Therefore, from the above explanation, the correct option is [A] 6 p-p single bond and [C] 4 lone pair of electrons
Additional information:
White phosphorus is very reactive because ${p_4}$ rings are held together by physical bonding but no chemical bonding.
These ${p_4}$ rings are in unstable arrangement.
The red phosphorus consists of complex chain structure formed by opening of ${p_4}$cage.

Black phosphorus has a layered structure and most thermodynamically stable form.
Phosphorus can be considered as light solid.
White phosphorus is very reactive and spontaneously ignites at ${30^\circ }C$ in moist air so usually it is stored under water. So that exposure to air can be prevented.
Red phosphorus is stable at room temperature but can be converted to white phosphorus by heat, sunlight or friction.
Note: White phosphorus crystallizes in a cubic system. Phosphorus atomic size is more so it can form a single bond with other phosphorus atoms. Hence it exists as tetra atomic molecules.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




