Chemistry Class 12 Chapter 6 Questions and Answers - Free PDF Download
FAQs on NCERT Solutions For Class 12 Chemistry Chapter 6 General Principles And Processes Of Isolation Of Elements - 2025-26
1. Is Chapter 6, General Principles and Processes of Isolation of Elements, in the CBSE Class 12 Chemistry syllabus for 2025-26?
No, as per the updated CBSE curriculum for the 2025-26 academic session, Chapter 6, 'General Principles and Processes of Isolation of Elements', has been removed from the Class 12 Chemistry syllabus. The NCERT solutions for this chapter are primarily for reference, competitive exams like JEE/NEET, or students following older syllabi.
2. If this chapter is deleted from the syllabus, why are NCERT solutions for it still useful?
While not required for CBSE board exams, the concepts in this chapter are foundational for competitive exams. The NCERT solutions for Chapter 6 are highly valuable for students preparing for JEE (Main and Advanced) and NEET, as questions related to metallurgical processes frequently appear in these tests. They provide a clear, step-by-step understanding of core principles like ore extraction and refining.
3. What key topics do the NCERT Solutions for Chapter 6 cover?
The NCERT solutions for this chapter provide detailed explanations and solved problems for several core metallurgical concepts. The main topics covered in the exercises include:
- Concentration of Ores: Methods like hydraulic washing, magnetic separation, froth flotation, and leaching.
- Extraction of Crude Metal: Processes like calcination, roasting, and the application of thermodynamic principles (Ellingham diagram).
- Refining of Metals: Techniques such as distillation, liquation, electrolytic refining, zone refining, and vapour phase refining.
- Thermodynamic and Electrochemical Principles: Understanding the feasibility of reduction reactions.
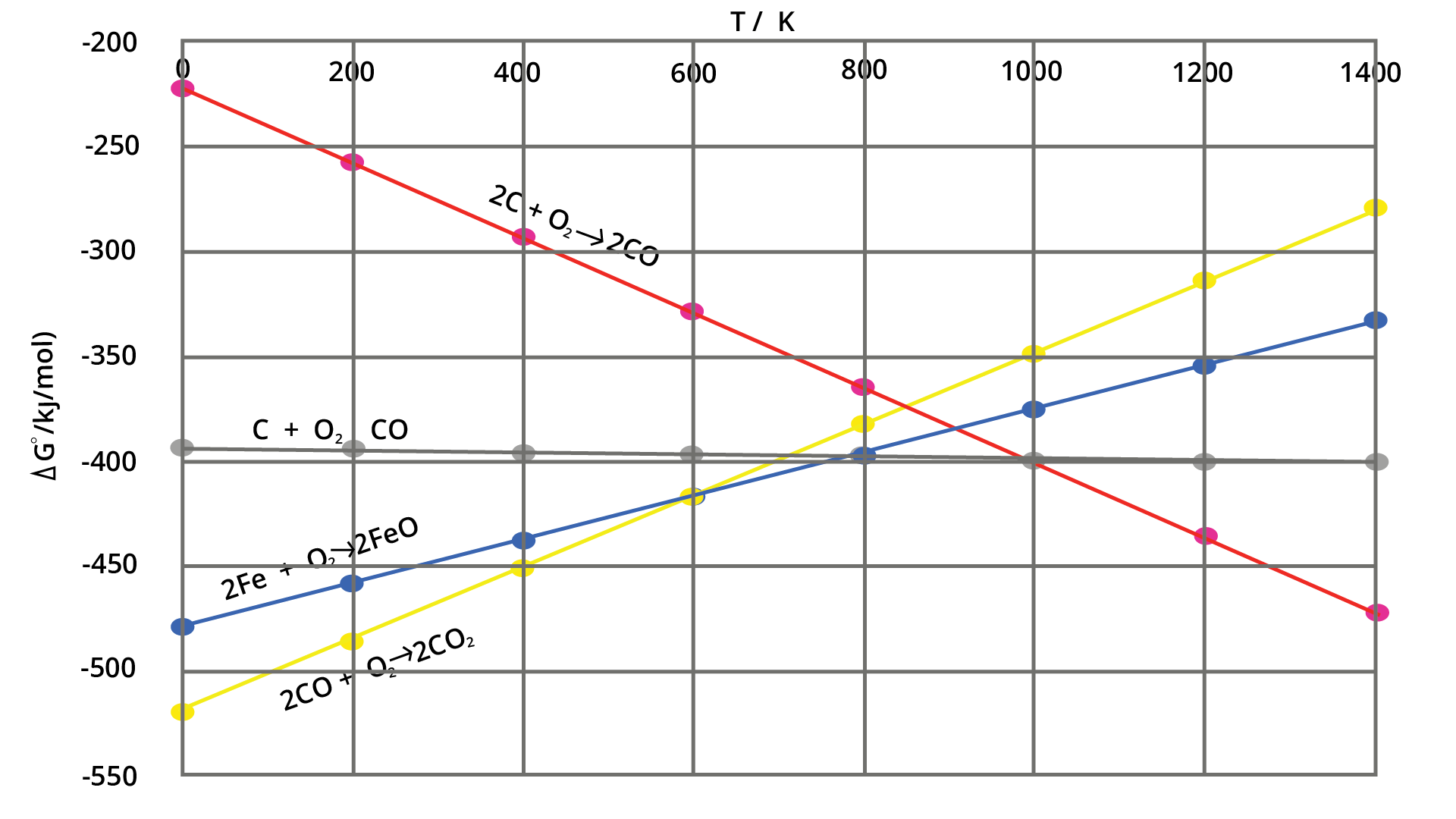
4. How do the NCERT Solutions help in solving problems based on the Ellingham diagram?
The NCERT solutions explain how to correctly interpret and apply the Ellingham diagram. They demonstrate the step-by-step method to determine the feasibility of reducing a metal oxide with a specific reducing agent at a given temperature. The solutions clarify that a metal can reduce the oxide of another metal if its Gibbs free energy of formation (ΔG°) is more negative at that temperature, which is visually represented by its line being lower on the diagram.
5. What is the correct method for explaining froth flotation as per the NCERT solutions?
According to the NCERT solutions, the correct method to explain the froth flotation process involves these key steps: First, a suspension of the powdered ore is made with water. Collectors (like pine oils) are added to enhance the non-wettability of the mineral particles. Froth stabilisers (like cresols) are used to sustain the froth. The key principle is that mineral particles are wetted by the oil and rise to the surface with the froth, while gangue particles are wetted by water and settle down.
6. How do the NCERT solutions for Class 12 Chemistry Chapter 6 differentiate between calcination and roasting?
The solutions clearly distinguish between the two processes based on the presence of air. Calcination is the process of heating an ore strongly, either in a limited supply of air or in its absence, primarily to remove volatile impurities and convert carbonate ores to oxides. In contrast, roasting involves heating the ore in a regular supply of air at a temperature below its melting point, a method typically used for sulphide ores to convert them into oxides.
7. Why is it important to understand thermodynamic principles when solving Chapter 6 exercises?
Understanding thermodynamic principles, particularly Gibbs free energy (ΔG), is crucial because it governs the spontaneity of a metallurgical reaction. The NCERT solutions emphasise that for any reduction process to be feasible, the change in Gibbs free energy must be negative. This helps in selecting a suitable reducing agent and the optimal temperature for the extraction of a metal from its ore, forming the basis for the Ellingham diagram.
8. What step-by-step approach do the NCERT solutions provide for problems on electrolytic refining?
The NCERT solutions outline a clear, step-by-step approach for electrolytic refining problems. The method involves:
- Identifying the impure metal, which is always made the anode.
- Using a strip of the pure metal as the cathode.
- Selecting a suitable electrolyte, which is a soluble salt of the same metal.
- Explaining that upon passing current, the pure metal from the anode dissolves into the electrolyte and deposits at the cathode, while less reactive impurities settle below the anode as anode mud.