Aldehydes Ketones and Carboxylic Acids Revision Notes Free PDF for NEET Preparation
Aldehydes Ketones and Carboxylic Acids are three prime classifications of organic compounds covered in Class 12 Organic Chemistry. This chapter explains what type of organic compounds they are and what features these compounds display. The presence of particular functional groups affects the classification of these compounds as aldehydes, carboxylic acids, and ketones. To understand the fundamental concepts of this chapter, download and refer to the Aldehyde Ketone and Carboxylic Acid notes for free from Vedantu.
These revision notes have been prepared in a concise and simple format so that the NEET aspirants can use them to revise this chapter effectively. It will become much easier for the students to study and recall the fundamental concepts by using these notes.
NEET Revision Notes Chemistry Aldehyde, ketone and Carboxylic acid
Aldehydes and ketones are carbonyl compounds, which means they are organic substances. The general formula for aldehydes is RCHO, while the general formula for ketones is ${\rm{RCOR'}}{\rm{.~ C}} = {\rm{O}}$ is the common group for carbonyl compounds.

Structure of Carbonyl Group

Structure of Carbonyl Group in Aldehyde

Structure of Carbonyl Group in Ketone
Ketones and aldehydes are employed as solvents, starting materials, and reagents in the chemical industry to synthesize other products.
Ketones dissolve a wide range of organic materials, have convenient boiling points for easy distillation, and have low toxicities. Formaldehyde is well known as the formalin solution used to preserve biological specimens and is also used in the formation of several important polymers such as Bakelite.
Many other ketones and aldehydes are utilized in foods, pharmaceuticals, and other items as flavorings and additives.
Almond extract is primarily made up of benzaldehyde, and carvone provides spearmint chewing gum with its minty flavor.
Physical Properties
Through coplanar sigma bonds aligned about 120 degrees apart, the carbonyl carbon atom is sp2 hybridized and connected to three additional atoms. To form a pi bond, the unhybridized p orbital coincides with an oxygen "p" orbital.
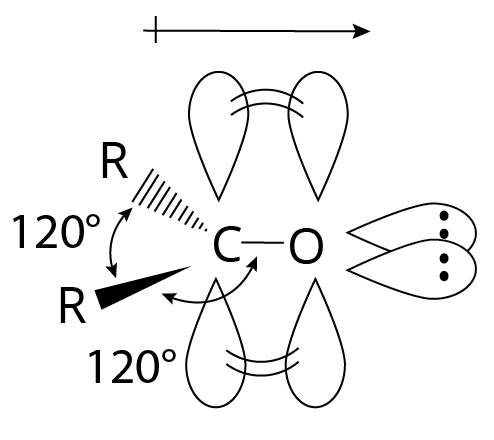
Structure of Carbonyl Group
Functional Group | Length | Energy |
Ketone C=O bond | $1.23{\rm{ }}\mathop {\rm{A}}\limits^\circ$ | 745 kJ/mol |
Alkene C=O bond | $1.34{\rm{ }}\mathop {\rm{A}}\limits^\circ$ | 611 kJ/mol |
1. Dipole Moment
Since oxygen is more electronegative than carbon and the bonding electrons are not shared equally, the carbonyl group's double bond has a significant dipole moment. Ketones and aldehydes have greater dipole moments than most alkyl halides and ethers because the less firmly bound help pi electrons are drawn more strongly toward the oxygen atom. We can use resonance forms to represent the unequal distribution of pi electrons.
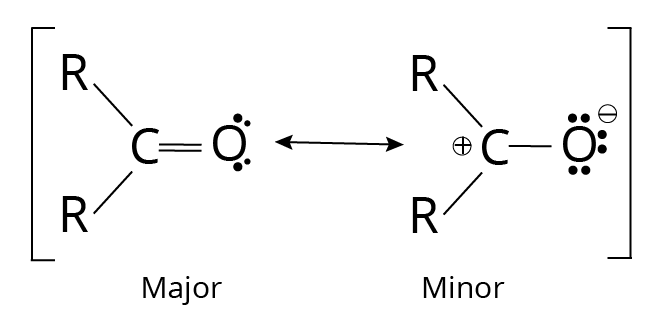
Resonance forms of Carbonyl Group
Because it involves more bonds and less charge separation, the first resonance form is more critical. The significant dipole moments of the ketones and aldehydes are illustrated here to demonstrate the contribution of the second structure.

Structure of Acetaldehyde and Acetone
Lewis acid is formed when a positively polarized carbon atom works as an electrophile and negatively polarized oxygen acts as a nucleophile (Lewis base)
2. Boiling Point
Ketones and aldehydes lack O-H and N-H bonds, they are unable to create hydrogen bonds with one another. As a result, their boiling points are lower than those of similar molecular weight alcohols.
The ketone and aldehyde are more polar and have a higher boiling point than the ether and alkane, but are less polar and have a lower boiling point than the hydrogen-bonded alcohol.

Boiling Point of Ketone and Aldehyde
3. Solubility
Although pure ketones and aldehydes are unable to form hydrogen bonds with one another, they do have lone pairs of electrons and can act as hydrogen bond acceptors for other compounds that have O-H or N-H bonds. The unshared electrons on a carbonyl oxygen atom can establish a hydrogen bond with the -OH hydrogen of water or alcohol, for example.
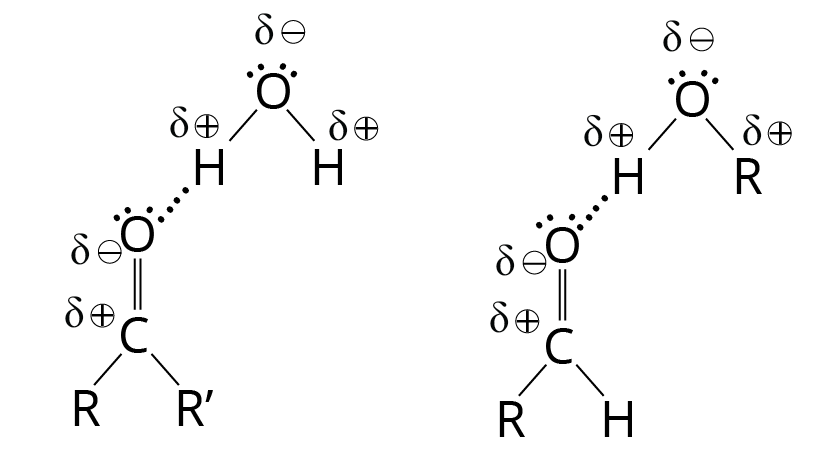
Linkage of Ketone and Aldehyde with Water
Ketones and aldehydes are good solvents for polar hydroxylic compounds like alcohols because of this hydrogen bonding. They're also highly water-soluble. Acetaldehyde and acetone are miscible with water (that is, they are soluble in any concentrations). Water is soluble in other ketones and aldehydes with up to four carbon atoms. These qualities of solubility are comparable to those of ethers and alcohols, which form hydrogen bonds with water as well.
Preparation of Carbonyl Compound

Preparation of Carbonyl Group
1. Alkanes
Carbonyl compounds are formed through the controlled oxidation of alkanes at high temperatures and pressures in the presence of a catalyst.
Example:
Image: Oxidation of alkane
2. Alkenes
Ozonolysis
If alkenes are generated using O3 and then reduced with Zn/H2O, they can be oxidized to aldehydes and ketones. The aldehyde produced cannot be separated if a reducing agent is not applied since it is further oxidized to carboxylic acids.
Example:

Ozonolysis of Alkene
Oxidative Cleavage
Strong oxidizing agents, such as acidic
${{\rm{K}}_{\rm{2}}}{\rm{C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}}$ or alkaline ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$, promote double bond oxidation, resulting in ketones and carboxylic acids.
Example:
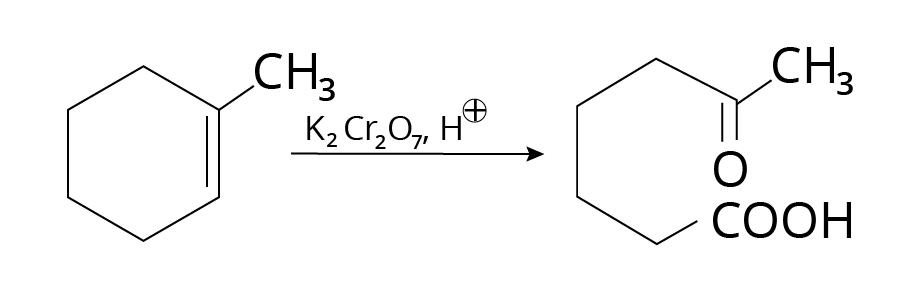
Oxidation Cleavage
Wacker Process
It's how acetaldehyde is made in the industry.
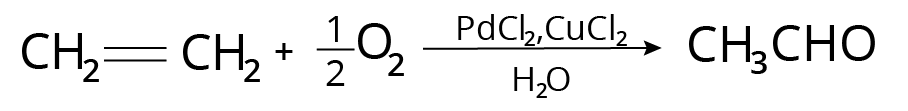
Wacker Process
Oxo Process
Hydroformylation is another name for this process. Under pressure, carbon monoxide and hydrogen are passed over a catalyst.
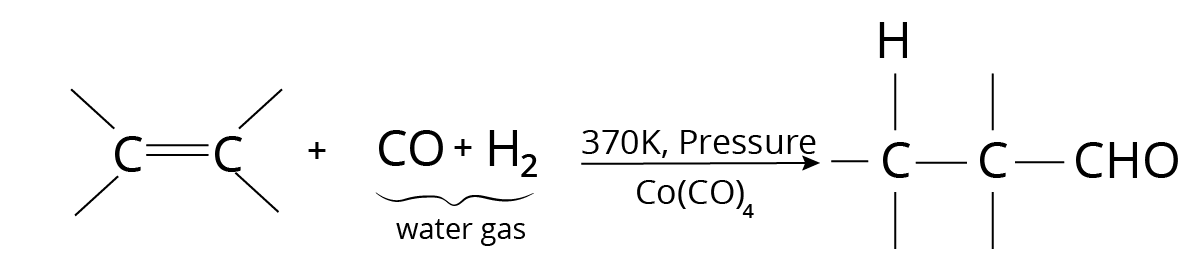
Hydroformylation Reaction
The resulting product is a mixture of isomeric and branched-chain aldehydes.
3. Alkynes
Acid Catalysed hydration in presence of Mercuric salt
This reaction occurs after the Markovnikov addition.
Example:
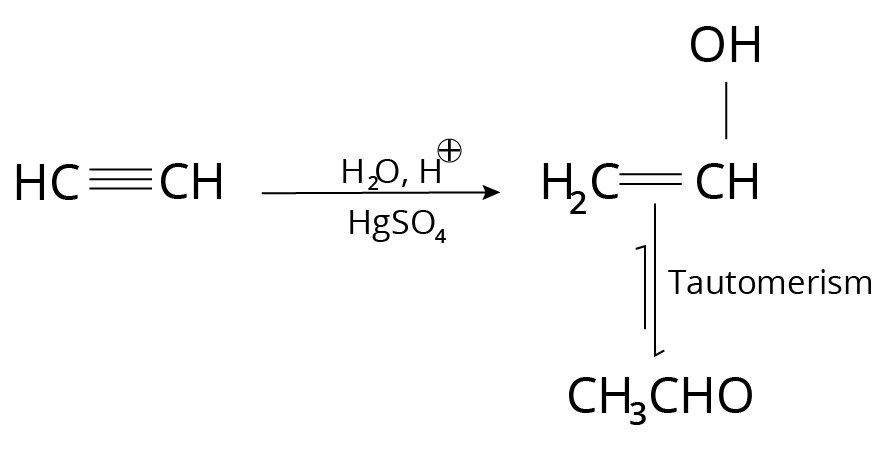
Reaction of Alkynes with Mercuric Salt
4. Alkyl Halides
Carbonyl compounds are formed when geminal dihalides are hydrolyzed.
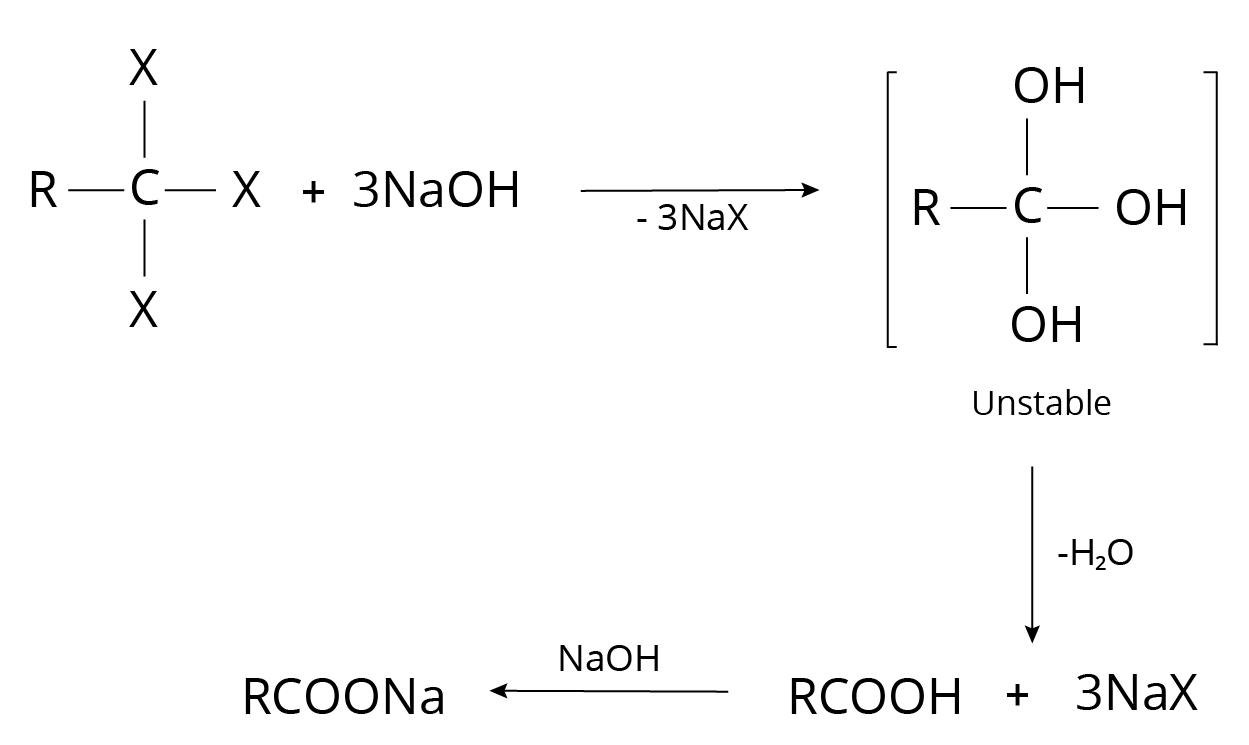
Reaction of Alkyl Halide
5. Alkyl Cyanides
Reduction of Cyanides
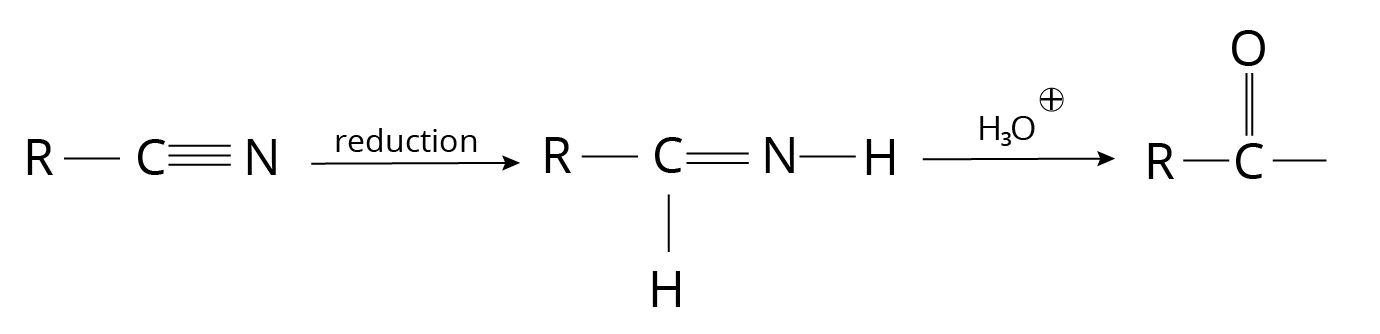
Reaction of Cyanides
1. The reaction is known as Stephan's Reduction when it is carried out with ${\rm{SnC}}{{\rm{l}}_{\rm{2}}}{\rm{, HCl}}{\rm{.}}$
2. DIBAL-H can also be used for the reduction (Di-Isobutyl Aluminium Hydride)
3. This approach produces only aldehydes.
6. Alcohols
Using proper reagents, primary alcohols (1°) are oxidized to aldehydes, whereas secondary alcohols (2°) are oxidized to ketones. The following procedures will not oxidize tertiary alcohols (3°).
Manganese dioxide or ${\rm{Mn}}{{\rm{O}}_{\rm{2}}}$
${\rm{Mn}}{{\rm{O}}_{\rm{2}}}$ oxidizes allylic, benzylic, and propargylic alcohol to aldehydes and ketones.
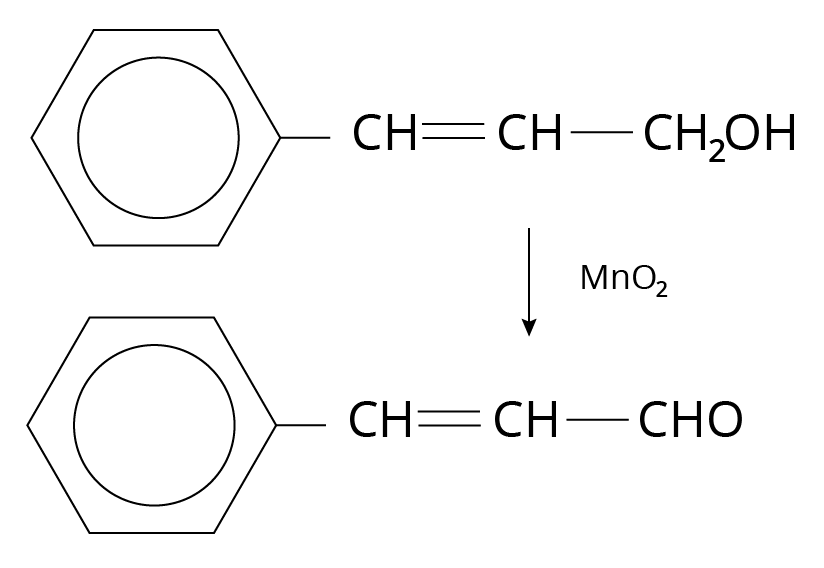
Oxidation of Alcohols
7. Carboxylic Acids and Derivatives
Decarboxylation of Ca Salts
Heating a calcium salt of monocarboxylic acids produces ketone. The result is an aldehyde when heated with the calcium salt of formic acid.
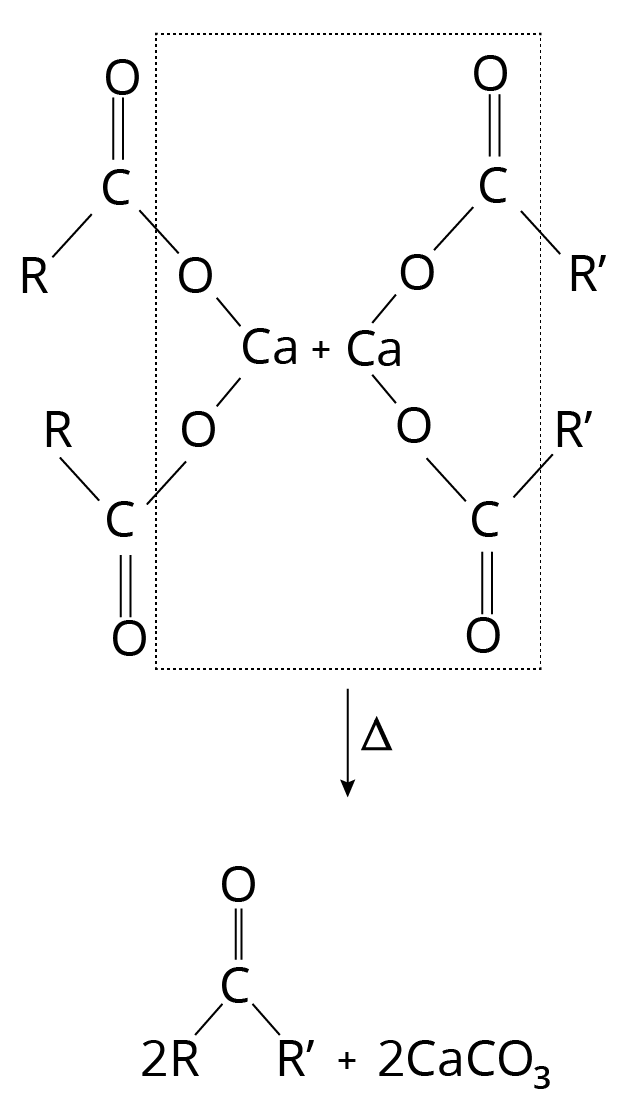
Decarboxylation Reaction
Rosemund’s Reaction
Palladium catalyst supported on barium sulphate is used to reduce acid chloride in boiling xylene. For intense poisoning, small amounts of quinoline and sulphur are also added. Acid chloride can also be converted to the aldehyde using lithium t-butoxyaluminium hydride.
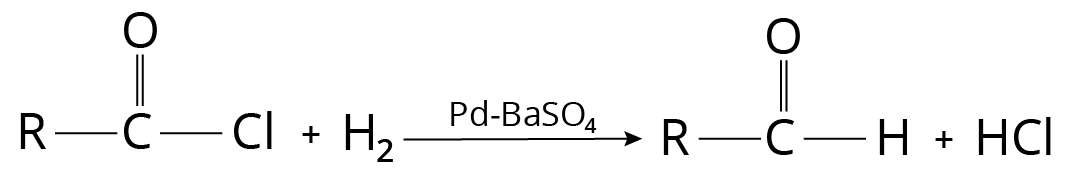
Rosemund’s Reaction
Reactions of Carbonyl Compounds

Reactions of Carbonyl Compounds
1. Addition Reactions
General Mechanism
'A tetrahedral carbonyl compound is formed from a trigonal carbonyl compound. The electronic and steric factors indicate that aldehyde is more reactive than ketone.
Addition of ${{\rm{H}}_{\rm{2}}}{\rm{O}}$- Hydrate Formation
Hydrate is a geminal diol that is usually unstable, which is why it can't be separated from the water. The equilibrium constant, which indicates the stability of the corresponding hydrate, affects formation.
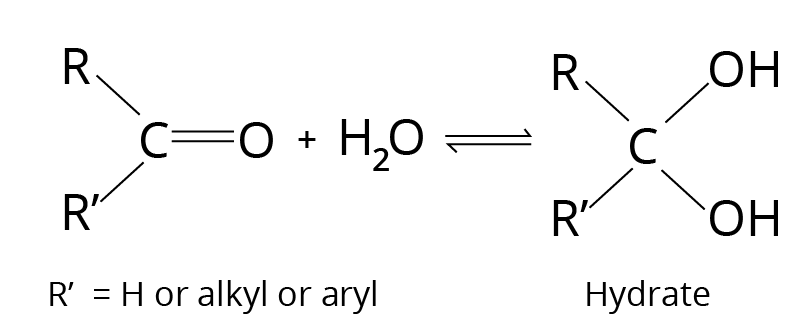
Addition Reaction
The hydrate is stabilized by electron-withdrawing groups.
2. Reduction Reactions
Reduction to Alkanes
(A) Wolff-Kishner Reduction
When hydrazone or semicarbazone is heated to 180°C with sodium ethoxide, nitrogen and hydrocarbons are released.
(B) Clemmensen Reduction
With an excess of the amalgamated zine (Zn-Hg) and HCl, aldehydes and ketones that are not acid sensitive give rise to the corresponding alkanes.
3. Oxidation Reactions
Aldehydes are powerful reducing agents because they are easily oxidised. Fehling's solution (an alkaline solution of ${\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}$ with tartaric acid) is reduced to the red cuprous oxide by aldehydes, while Tollen's reagent (ammoniacal silver nitrate) is reduced to metallic silver by aldehydes. ${\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}$ salt is not reduced by benzaldehyde (Fehling solution). Ketones do not decrease Tollen's reagent or the Fehling solution (except a-hydroxy ketones).
Tollen's test is positive when benzaldehyde is present. Permanganate, acid dichromates, and other oxidizing agents oxidize all aldehydes and ketones. These powerful oxidizers oxidize nearby carbon atoms in ketonic carbonyls, leaving the carbonyl group primarily with smaller alkyl groups (Popoff’s rule).
4. Alpha Substitutions
A hydrogen atom at the alpha carbon atom (the carbon adjacent to the carbonyl group) is replaced by another group in alpha replacements. Because the 'enolate ion that arises from its removal is resonance-stabilized, with the negative charge delocalized over the alpha carbon atom and the carbonyl oxygen atom, the alpha hydrogen is more acidic.
Haloform Reaction
'Methyl ketones are cleaved in the presence of a base when they react with excess halogen. A trihalomethane (haloform) and a carboxylate salt are the end products. The rate has been found to be independent of bromine concentration in aqueous sodium hydroxide. The reaction is used to determine the structure of things. In most cases, the positive iodoform (bright yellow ppt) confirms as part.
5. Special Reactions
Aldol Condensation
(A) Self- Aldol Condensation
(At or below room temperature) dimerizes and produces alcohol.
The reaction includes the nucleophilic addition of an enolate ion to the carbonyl group., $\alpha,\beta$ unsaturated aldehyde or ketone is the result of the subsequent dehydration. Aldehydes are better than ketones for aldol condensation.
(B) Cross- Aldol Condensation (Claisen – Schmidt Reaction)
The most suited is a mixed aldol condensation, specifically the condensation of aromatic aldehyde (or aldehyde without $\alpha$-Hs) with an enolizable aldehyde and ketone.
(C) Intramolecular Aldol Condensation
This reaction occurs when dicarbonyl compounds undergo cyclization; the product generated is dependent on the size of the ring (usually 5 or 6 membered rings are formed).
Analysis of Aldehydes and Ketones
The addition of nucleophilic reagents to the carbonyl group of aldehydes and ketones, particularly ammonia derivatives, is used to describe them. For example, an aldehyde or ketone reacts with 2, 4-nitrophenythydrazine to produce an insoluble yellow or red solid.
Aldehydes are distinguished from ketones by their ease of oxidation: aldehydes pass the Tollens' reagent test, but ketones do not. Some phenols and amines also provide positive Tollen's tests; however, these compounds do not give positive tests with 2, 4-dinitrophenylhydrazine.
The Schiff test is an extremely sensitive aldehyde test. The fuchsin-aldehyde reagent combines with an aldehyde to produce a distinctive magenta color.
In CCI4, aliphatic aldehydes and ketones containing an alpha-hydrogen react with Br. This reaction is too slow to be mistaken with an unsaturation test, and it also liberates HBr.
The melting points of derivatives such as 2, 4-dinitrophenylhydrazones, ‘oximes, and semicarbazones are used to identify aldehydes and ketones.
The iodoform test is used to identify methyl ketones.
1. Iodoform Test
The iodoform test determines whether a ketone is a methyl ketone. A ketone of the structure is treated with iodine and sodium hydroxide (sodium hypoiodite, NaO).
2. Periodic Acid Oxidation
Compounds having —OH groups connected to adjacent carbon 'atoms undergo oxidation with the breaking of carbon-carbon bonds when exposed to periodic acid, HIO4.
Uses of Aldehyde
Bakelite is a chemical created when phenol and formaldehyde combine. Plastics, coatings, and adhesives all use bakelite in some capacity.
The chemical formaldehyde is necessary for various industrial processes, including embalming, making glue, tanning, and creating polymeric products.
It functions as a fungicide, an insecticide, and a germicide.
Drug testing is aided by formaldehyde. Photography also makes use of it.
The substance "acetaldehyde" can be used to make acetic acid and pyridine derivatives.
For the creation of perfumes, cosmetics, and colors, benzaldehyde (aldehyde) is a necessary ingredient. It is included to give certain food products an almond flavor. It serves as a bee deterrent as well.
Uses of ketone
For several types of plastics and synthetic fibers, ketone functions as an effective solvent.
Acetone can be used to remove nail polish and thin out the paint.
Additionally, it is employed medically to cure acne and perform chemical peeling procedures.
One of the often used solvents is butanone, also referred to as methyl ethyl ketone. It is employed in the manufacture of textiles, varnishes, paint removers, paraffin wax, and plastics, among other things.
Cyclohexanone, a crucial ingredient in the manufacture of nylon, is another significant ketone.
Carboxylic acid and Derivatives
Introduction
Compounds with the carboxyl functional group are known as carboxylic acids.
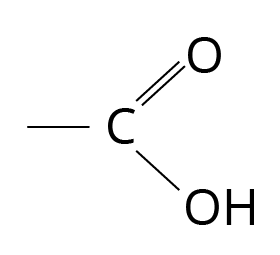
Structure of Carboxylic Acid
'Depending on whether the COOH group is connected to an aliphatic alkyl chain or an aryl group, the carboxylic acids can be aliphatic (R-COOH) or aromatic (Ar-COOH).
Polymers, medicines, solvents, and food additives are all made with carboxylic acids. Acetic acid (a component of vinegar and a precursor to solvents and coatings), acrylic and methacrylic acids (precursors to polymers and adhesives), adipic acid (polymers), citric acid (beverages), ethylenediaminetetraacetic acid (chelating agent), fatty acids (coatings), maleic acid (polymers), propionic acid (food preservatives), terephthalic acid (food preservatives). The major components of lipids are fatty acid esters, whereas the main components of proteins are polyamides of amino carboxylic acids.
Physical Properties and Acidic Character
1. Boiling Point
Carboxylic acids boil at far greater temperatures than similar molecular weight alcohols, ketones, or aldehydes.
2. Melting Point and Physical State
At normal temperature, aliphatic acids with up to nine carbon atoms are colorless liquids with foul odors. Because of their low volatility, the higher acids are 'wax-tike solids' and are nearly odorless. In aprotic and nonpolar solvents, they exist as dimers.
Unbranched acids with an even number of carbon atoms have a greater melting point than homologs with an odd number of carbon atoms. The differences in intermolecular attractive forces in the crystalline state explain the fluctuating behavior of the melting point.
3. Solubility
Carboxylic acids create hydrogen bonds with water, and lower-molecular-weight carboxylic acids (up to four carbon atoms) are water-miscible. Water solubility reduces as the length of the hydrocarbon chain rises until acids with more than 10 carbon atoms are essentially insoluble in water.
4. Acidity of Carboxylic Acids
Carboxylate ions have two equivalent canonical structures A and B. Because carboxylate ions are more stable, the carboxylic acid is acidic in nature because the chemical equilibrium favors the creation of stable ions.
Preparation of Carboxylic Acids

Preparation of Carboxylic Acid
1. Alkanes
At 120°C, long-chain alkanes are oxidized by air in the presence of a manganese acetate or manganous stearate catalyst.
2. Alkenes
Ozonolysis
On oxidative ozonolysis, monosubstituted alkenes produce carboxylic acid.
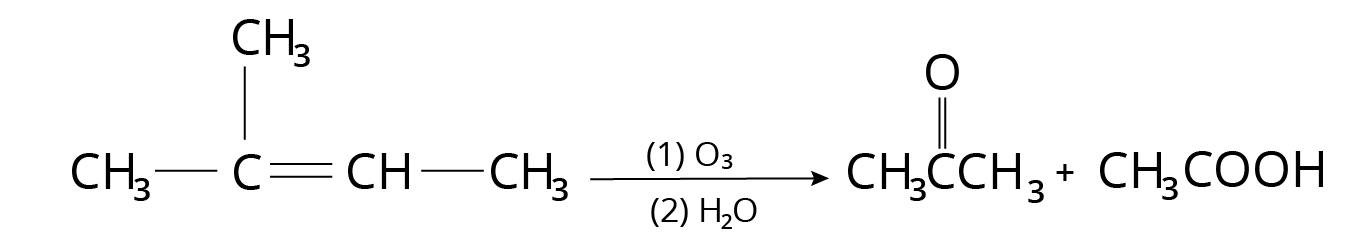
Ozonolysis Reaction
3. Alkynes
Ozonolysis
On ozonolysis, alkynes produce carboxylic acids.
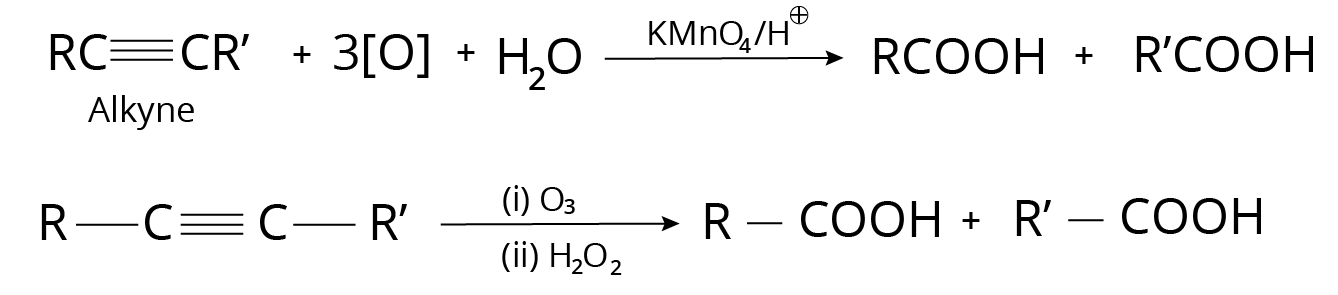
Reaction with Alkynes
4. Alkyl Halides
Hydrolysis of terminal tri-halogen derivatives of Alkanes
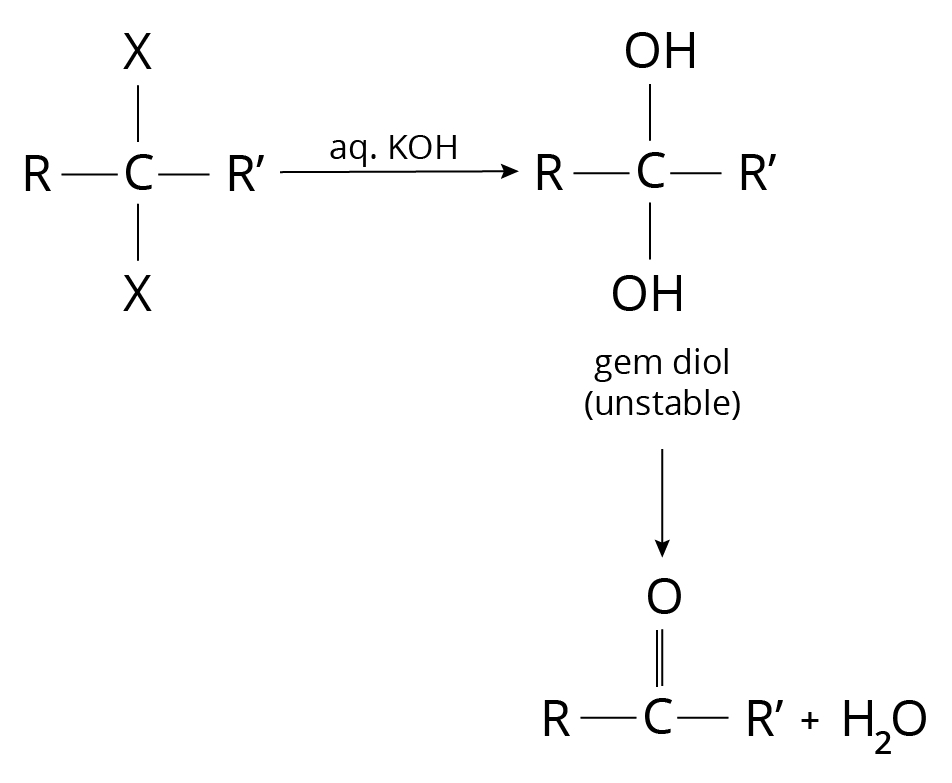
Reaction of Alkyl Halide
5. Alcohols
Carboxylic acids are formed when primary alcohols are oxidized.
Example:
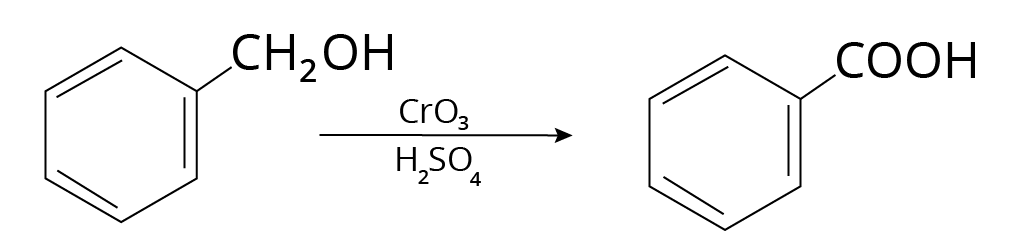
Reaction of Alcohols
6. Aldehydes and Ketones
Oxidation
Under aggressive circumstances, such as heated ${{\rm{K}}_{\rm{2}}}{\rm{C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}}{\rm{/}}{{\rm{H}}^{\rm{ + }}}$, ketones oxidize to produce a mixture of acids.
7. Carboxylic Acids Derivatives
On acidic or basic hydrolysis, carboxylic acids are formed from acyl halide, carboxylic ester, anhydride, and amide.
Reactions of Carboxylic Acid

Reactions of Carboxylic Acid
1. Reactions as an Acid
Reactions with strongly Electropositive Metals
Monocarboxylic acids react with strong electropositive metals (such as Na, K, Zn, and others) to produce hydrogen and salts (acidic character).
${\rm{2RCOOH}} + {\rm{Na}} \to {\rm{2RCOONa}} + {{\rm{H}}_{\rm{2}}}$
Reaction with Alkali
Monocarboxylic acids react with strong electropositive metals (such as Na, K, Zn, and others) to produce hydrogen and salts (acidic character).
${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COOH}} + {\rm{NaOH}} \to {\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COONa}} + {{\rm{H}}_{\rm{2}}}{\rm{O}}$
When these salts are exposed to dilute mineral acids, they regenerate carboxylic acids.'
${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COONa}} + {\rm{HCl}} \to {\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COOH}} + {\rm{NaCl}}$
Reaction with Carbonates and Bicarbonates
Due to CO effervescence, this reaction is a laboratory test of carboxylic group (-COOH).
$\begin{array}{l}{\rm{2C}}{{\rm{H}}_{\rm{3}}}{\rm{COOH}} + {\rm{N}}{{\rm{a}}_{\rm{2}}}{\rm{C}}{{\rm{O}}_{\rm{3}}} \to 2{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COONa}} + {\rm{C}}{{\rm{O}}_{\rm{2}}} + {{\rm{H}}_{\rm{2}}}{\rm{O}}\\{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COOH}} + {\rm{N}}{{\rm{a}}}{\rm{HC}}{{\rm{O}}_{\rm{3}}} \to {\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COONa}} + {\rm{C}}{{\rm{O}}_{\rm{2}}}{\rm{ + }}{{\rm{H}}_{\rm{2}}}{\rm{O}}\end{array}$
2. Nucleophilic Acyl Substitution
Formation of Esters
Esterification is the name for this reaction, which is catalysed by a little amount of inorganic acids.
The C-OH bond of the acid and the O-H bond of the alcohol are broken during esterification.
Lactones are cyclic esters formed by the intramolecular esterification of hydroxy acids.
3. Reduction
Reduction to Alcohols
${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$ in ether solution or with ${{\rm{H}}_{\rm{2}}}$ in the presence of a copper chromite ${\rm{CuC}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{4}}}$ catalyst or ${\rm{B}}{{\rm{H}}_{\rm{4}}}{\rm{, THF}}$ ( diborane or ${{\rm{B}}_{\rm{2}}}{{\rm{H}}_{\rm{6}}}$)
4. Carboxyl Group Reactions
Decarboxylation
When a carboxylic acid's anhydrous alkali salt is heated with soda lime (NaOH:CaO in a 3: 1 ratio), an alkane is produced.
Carbanion intermediate is used in the reaction. The rate of decarboxylation is proportional to the stability of the carbanion.
Uses of Carboxylic Acid
Higher fatty acids are required for soap production. Most commonly, higher fatty acids like stearic acid are salts of sodium or potassium in soaps.
Numerous organic acids are employed by the food sector in the production of soft drinks, culinary items, etc. For instance, vinegar is made using acetic acid. Preservatives use sodium salts of organic acids.
Numerous medications in the pharmaceutical business use organic acids.
When making rubber, acetic acids are frequently employed as a coagulant.
Organic acids are widely used in the production of rayon, fragrances, and dyes.
Solved Examples:
1. The correct order of increasing acidic strength is _____________.
Phenol < Ethanol < Chloroacetic acid < Acetic acid
Ethanol < Phenol < Chloroacetic acid < Acetic acid
Ethanol < Phenol < Acetic acid < Chloroacetic acid
Chloroacetic acid < Acetic acid < Phenol < Ethanol
Correct Option: (C)
Explanation:
When it comes to ethanol, the obtained cation is not stabilized by resonance. The phenoxide ion obtained is stabilized by resonance, phenol is more acidic than ethanol. Chloroacetic acid has a higher acidity than acetic acid. Carboxylic acids are acidic in comparison to alcohols and phenols. Thus, option (C) is correct.
2. Which of the following compounds will give butanone on oxidation with alkaline ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$ solution?
Butan-1-ol
Butan-2-ol
Both
None of these
Correct Option: (B)
Explanation:
Primary alcohol like Butan-1-ol on reaction with a strong oxidizing agent like ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$ gives carboxylic acid, whereas Secondary alcohol like Butan-2-ol gives ketone on oxidizing under the pressure of ${\rm{KMn}}{{\rm{O}}_{\rm{4}}}$. Thus, option B is correct.
Importance of Class 12 Chemistry Aldehydes Ketones and Carboxylic Acids
This is a crucial chapter of organic chemistry that teaches students about three different classes of organic compounds. It covers Aldehydes, Ketones, and Carboxylic Acids by explaining their chemical features and how they are prepared or formed.
All the reactions related to these compounds as reactants and products will be explained in detail by using respective formulas and chemical equations. Students will learn how the functional groups of these compounds behave in such chemical reactions.
The objective of studying these reactions is to understand the difference between the functional groups defining these compounds. If you look closely, you will find that all these groups involve oxygen as the prime constituent. An aldehyde contains a –CHO group. A ketone contains a C=O group and a carboxylic acid contains a –COOH group. These functional groups display characteristic features that project from the reactions they take part in.
This chapter will also explain the nomenclature of these compounds and their physical and chemical properties. It will also explain how these compounds are prepared following specific chemical reactions.
To understand the basic concepts related to these compounds and develop a strong foundation, refer to the Aldehydes Ketones and Carboxylic Acids notes PDF available on Vedantu.
Benefits of Aldehyde Ketone and Carboxylic Acid Notes for NEET
These revision notes are the ideal study material you can avail to revise this chapter within a short span of time. Once you are done preparing this chapter, you can download and refer to these notes to revise the fundamental concepts related to these organic compounds and the reactions involved.
It will also become easier to remember the steps of all the chemical reactions described involving these compounds as reactants or products with these revision notes.
The experts have purposefully developed an easier format so that students can memorize the definitions, descriptions, derivations of formulas, and reactions of this chapter during the NEET exam. It will help them to answer fundamental questions accurately.
The concise format of these revision notes will reduce your preparation time for this chapter and help you to complete the NEET Chemistry syllabus faster.
Download Free Aldehyde Ketone and Carboxylic Acid Notes PDF for NEET
You can avail of the Aldehydes Ketones and Carboxylic Acids Class 12 notes PDF download option for free and get the best study material for revising this vast chapter. Strengthen your conceptual foundation in organic chemistry with these revision notes. Learning and revising the concepts will not be a problem when you have these notes. It will help you to become more confident while answering questions to perform excellently in the NEET exam.
Other Important Links Related to NEET Aldehydes Ketones and Carboxylic Acids
Other Important Links for NEET Aldehydes Ketones and Carboxylic Acids |
NEET Aldehydes Ketones and Carboxylic Acids Important Questions |
NEET Chemistry Revision Notes - Chapter Pages
NEET Chemistry Chapter-wise Revision Notes | |
Classification of Elements and Periodicity in Properties Notes | |
Aldehydes Ketones and Carboxylic Acids Notes | |
FAQs on Aldehydes Ketones and Carboxylic Acids Notes for NEET Chemistry Revision
1. What are carboxylic acids?
Carboxylic acids are organic acids that contain a carboxylic group (-COOH) attached to one of the constituent carbon atoms of the molecule. Formic acid (HCOOH) is a common example of a naturally existing carboxylic acid.
2. What happens when primary alcohols are oxidized using strong reagents?
The carbon atom attached to the hydroxyl group (-OH) gets oxidized in presence of the strong oxidizing agents to form a carboxylic group (-COOH). It means that primary alcohol becomes a carboxylic acid on oxidation.
3. What is the difference between alcohol and aldehyde?
An aldehyde contains a (-CHO) group whereas alcohol contains a hydroxyl group (-OH).
4. What is ozonolysis?
The organic reaction where ozone is broken down to oxidize an organic compound is called ozonolysis.

























