Revision Notes on Hydrogen for NEET 2026 - Free PDF Download
The chapter titled Hydrogen is a crucial portion of the syllabus for the NEET entrance examination. This chapter focuses on delivering concepts related to the element Hydrogen, its properties, reactions, and isotopes. It also provides ample explanation on the different topics that are related to the chemical and physical properties of the element. In order to delve deeper into the chapter, students need to download Hydrogen class 11 notes.
The notes have been prepared by the experts at Vedantu and will help students understand the concepts of the chapter in an efficient way. The subject matter experts at Vedantu have left no detail unexplained in the revision notes. Using a simpler language, these Hydrogen class 11 notes for NEET become an incredible study source for students to learn about the chapter.
Revision Notes on Hydrogen for NEET 2026
Access NEET Revision Notes Chemistry Hydrogen
Occurrence:
The most common component within the universe is an element. Element makes up a big a part of the sun and different stars. in keeping with astronomers, element atoms compose ninetieth of the universe's atoms. The component element is concerned in additional compounds than the other. Water is the most prevailing element compound on the world. Petroleum, various minerals, polyose and starch, sugar, fats, oils, alcohols, acids, and dozens of different parts all contain elements.
Isotopes:
Hydrogen is a colourless, odourless, tasteless, and nonpoisonous gas made up of the diatomic molecule ${H_2}$ at room temperature. Protium, $1H$, deuterium, $2H$ (or "$D$"), and tritium, $3H$ (or "$T$") are the three isotopes of hydrogen, which, unlike other elements, have separate names and chemical symbols. There is one atom of deuterium for every $7000{\text{ }}H$atoms in a naturally occurring sample of hydrogen, and one atom of radioactive tritium for every $1018{\text{ }}H$ atoms. Because they have identical electron configurations, the chemical characteristics of the various isotopes are quite similar, but their physical qualities differ due to their varied atomic weights. The vapour pressure of deuterium and tritium is lower than that of common hydrogen. As a result, the heavier isotopes are concentrated in the final regions of liquid hydrogen to evaporate. Deuterium is produced through electrolysis of heavy water ${D_2}O$. The majority of tritium comes from nuclear processes.
Preparation of Hydrogen:
The following are the most popular hydrogen preparation methods
1. From Steam and Carbon or Hydrocarbons
The cheapest and most common source of hydrogen is water. Steaming coke (an impure form of elemental carbon) at 1000°C creates water gas, a combination of carbon monoxide and hydrogen:
By passing hydrocarbons from natural gas or petroleum and steam over a nickel-based catalyst, a combination of hydrogen and carbon monoxide can be produced. A hydrocarbon reactant like propane is can be considered as an example:
2. Electrolysis:
When direct current electricity travels through water containing an electrolyte such as H2SO4, hydrogen is formed. At the cathode, hydrogen bubbles develop, while oxygen evolves at the anode. The overall reaction is as follows:
3. Reaction of Metals with Acid:
This is the most practical way for creating hydrogen in the laboratory. Metals with lower reduction potentials create hydrogen gas and metal salts by reducing the hydrogen ion in dilute acids. In dilute hydrochloric acid, iron, for example, creates hydrogen gas and iron(II) chloride:
4. Reaction of Metal Hydrides with Water:
It is possible to make hydrogen by reacting hydrides of active metals with water, which include the extremely highly basic ${H^ - }$anion:
Properties of Hydrogen:
Physical Properties: In nature, $H$ gas is colourless, odourless, and tasteless. though it's a flammable gas, it doesn't promote combustion. it's lighter than air and water insoluble. it's atomic mass of $1.008{\text{ }}amu$ and a $1312{\text{ }}kJ{\text{ }}mo{l^{ - 1}}$ ionisation heat content.
Chemical Properties: The bond dissociation of total heat may be a major determinant of chemical characteristics. The gas molecule creates the $H - H$ bond, which has the best bond total heat of any element's atoms.
The basic structure of the hydrogen atom can be shown below:
Hydrogen gas is regarded as the clean fuel of the future, as it is made from water and oxidises back to the water. Fuel cells that use hydrogen are rapidly being viewed as "pollution-free" energy sources, and are currently being utilised in certain buses and autos.
Hydrogen has a wide range of applications. It is used to create ammonia for agricultural fertiliser (the Haber process) as well as cyclohexane and methanol, which are intermediates in the manufacturing of polymers and medicines. It's also used in the oil-refining process to remove sulphur from fuels. When hydrogenating oils generate fats, such as margarine, large amounts of hydrogen are utilised.
Hydrogen is employed as a protective environment in the glass industry for creating flat glass.
For manufacturing flat glass sheets, hydrogen is employed as a protective environment in the glass industry. It is utilised as a cleaning gas in the electronics sector during the fabrication of silicon chips.
Because of its low density, hydrogen was an obvious option for one of its earliest practical applications: filling balloons and airships. However, it has a strong reaction with oxygen (forming water), and its use in filling airships came to a stop when the Hindenburg caught fire.
Hydrides
The hydrogen anion, ${H^ - }$, is a negative hydrogen ion, or a hydrogen atom with an additional electron captured. The hydrogen anion is a key component of stars' atmospheres, such as the Sun's. This ion is known as hydride in chemistry.
Types of Hydride Ions:
The three primary forms of hydrides are saline hydride or ionic hydride, metallic hydride, and covalent hydride, which are characterised by the type of chemical bond involved. The structure of the fourth type of hydride, the dimeric hydride (of which borane, $B{H_3}$, is an example), can also be used to distinguish it.
1. Saline Hydrides or Ionic Hydrides:
The existence of hydrogen as a negatively charged ion (i.e. ${H^ - }$, ) defines saline, or ionic, hydrides. The hydrides of alkali metals and alkaline earth metals are sometimes referred to as saline hydrides (with the possible exception of beryllium hydride, $Be{H_2}$, and magnesium hydride, $Mg{H_2}$). At high temperatures ($30-700{\text{ }}^\circ C{\text{ }}[570-1300{\text{ }}^\circ F]$), these metals react directly with hydrogen to generate hydrides with the general formulae $MH$ and $M{H_2}$. When pure, these compounds are white crystalline solids, however, they are frequently grey due to trace metal impurities.
Beryllium and magnesium, both alkaline-earth metals, generate stoichiometric $M{H_2}$ hydrides that are less ionic and more covalent than the other alkaline-earth metals hydrides. The transition metals and inner transition metals may create a wide range of hydrogen-based compounds, from simple stoichiometric systems to exceedingly complex non-stoichiometric complexes. (In contrast to stoichiometric compounds, which have a fixed composition, non-stoichiometric compounds have a changeable composition.)
2. Metal Hydrides:
Metallic hydrides are created when hydrogen gas is heated with metals or alloys. Compounds of the most electropositive transition metals have been investigated the most (the scandium, titanium, and vanadium families). Titanium ($Ti$), zirconium ($Zr$), and hafnium ($Hf$), for example, generate nonstoichiometric hydrides when they absorb hydrogen and release heat. These hydrides show chemical reactivity comparable to that of the finely split metal, being stable at room temperature but reactive when heated in air or with acidic chemicals. They have a metal-like appearance, with greyish black solids. The metal appears to be in a $ + 3$ oxidation state, with mostly ionic bonding.
3. Covalent Hydrides:
Covalent hydrides are mostly hydrogen-non-metal complexes in which the bonds are clearly electron pairs shared by atoms with similar electro-negativities. Most non-metal hydrides, for example, are volatile compounds kept together in the condensed form by weak intermolecular van der Waals interactions. Covalent hydrides are liquids or gases with a low melting point and a high boiling point, unless their characteristics are altered by hydrogen bonding (as in water). Boron ($B$), aluminium ($Al$), and gallium ($Ga$) from Group 13 of the Periodic Table can be used to make covalent hydrides. Both boron ($B{H_4}$) and aluminium ($Al{H_4}$) ionic hydrogen species are widely employed as hydride sources.
Water:
${H_2}O$, It is made up of two elements: hydrogen and oxygen. Water is formed when two hydrogen molecules interact with one oxygen molecule. It comes in three different forms: solid, liquid, and gas (or vapour). It has the unique ability to dissolve a wide range of other chemicals, making it a universal solvent.
Structure of Water:
The molecular structure of water is bent. Oxygen is joined to two hydrogen atoms. The shared pair of electrons is drawn to chemical element atoms a lot of powerfully than gas atoms i.e. oxygen, leading to dipole formation. Oxygen element atoms develop partial negative charges, whereas hydrogen atoms acquire partial positive charges. The angle of the $H - O - H$ bond is ${104.5^ \circ }$. A perfect $s{p^3}$ hybridized atomic orbital features a bond angle that's somewhat narrower. Its original symmetry is tetrahedral.
Physical Properties of Water:
Chemical Properties of Water:
1. Amphoteric Nature of Water:
One of the most significant characteristics of water is its amphoteric tendency. The capacity to operate as an acid or base is referred to as amphoteric. Water has neither an acidic nor a basic pH in its natural form. Its capacity to give and absorb protons is the primary reason for its existence. Rainwater, on the other hand, has a pH of $5.2 - 5.8$, making it mildly acidic.
Water as Acid:
${{\text{H}}_{\text{2}}}{\text{O + N}}{{\text{H}}_{\text{3}}}{\text{ }}\overset {} \leftrightarrows {\text{ NH}}_{\text{4}}^{\text{ + }}{\text{ + O}}{{\text{H}}^{\text{ - }}}$
${\text{acid base }}$
Water as Base:
${{\text{H}}_{\text{2}}}{\text{O + }}{{\text{H}}_{\text{2}}}{\text{S }}\overset {} \leftrightarrows {\text{ }}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{ + H}}{{\text{S}}^{\text{ - }}}$
${\text{base acid}}$
2. Self-ionisation Property:
Water is ionised during the auto-protolysis process, which implies that in order to generate the hydroxide ion $O{H^ - }{\text{ and }}{{\text{H}}_{\text{2}}}{\text{O}}$ deprotonates (proton removal).
$2{H_2}O \Leftrightarrow {H_3}{O^ + } + O{H^ - }$
3. Hydrolysis Reaction:
Water has a high dielectric constant, which indicates it has a strong inclination to hydrate. Water exhibits significant interactions with salt ions when it is surrounded by hydration shells.
$SiC{l_4} + 2{H_2}O \to Si{O_2}4HCl$
4. Redox Reaction:
Water is a major supply of dihydrogen, which may be decreased when it reacts with a highly electropositive metal such as Sodium.
${H_2}O + Na \to 2NaOH + {H_2}$
There are Two Types of Water:
Hard water comprises calcium ($Ca$) and magnesium ($Mg$) salts in the form of hydrogen carbonate, chlorides, and sulphates. Water that is free from calcium and magnesium salts is known as soft water.
Heavy Water:
Because it is made up of deuterium, heavy water is also known as ${D_2}O$ in chemistry. Deuterium is a hydrogen isotope. Heavy water is used as a moderator in nuclear reactors to slow neutrons down so that they react more with fissile uranium$ - 235$ than uranium$ - 238$. Heavy water cannot be consumed and is unfit for human consumption. The metabolic processes in a person's body will slow down if he consumes a lot of water.
Physical Properties:
Heavy water, like ordinary water, is a colourless, odourless, and tasteless mobile liquid. Because heavy water has a higher molecular weight ($20g$) than water ($18g$), its boiling and freezing points, specific heat, density, viscosity, temperature of maximum density, and latent heat of vaporisation are all higher in heavy water than in water. The dielectric constant of heavy water, on the other hand, is smaller than that of water. As a result, ionic compounds dissolved in heavy water have a lower solubility than those dissolved in water.
Chemical Properties of Heavy Water:
Heavy water may participate in any chemical reaction that water can. Heavy water, on the other hand, takes longer to respond than ordinary water. Because the O–D bond has a larger dissociation energy than the O–H bond, heavy water has a lower reactivity than water.
1. Deuterolysis:
Water hydrolyses many inorganic salts. When heavy water is utilised, similar processes known as salt Deuterolysis occur.
${\mathbf{AlC}}{{\mathbf{l}}_{{\mathbf{3}}\;}} + {\text{ }}{\mathbf{3}}{{\mathbf{D}}_{\mathbf{2}}}{\mathbf{O}}{\text{ }} \to {\text{ }}{\mathbf{Al}}{\left( {{\mathbf{OD}}} \right)_{{\mathbf{3}}\;}} + {\text{ }}{\mathbf{3DCl}}$
2. Action on Metals:
Deuterium is released when heavy water combines with reactive metals like sodium and calcium, resulting in heavy alkalis.
${\mathbf{2Na}}{\text{ }} + {\text{ }}{\mathbf{2}}{{\mathbf{D}}_{\mathbf{2}}}{\mathbf{O}}{\text{ }} \to {\text{ }}{\mathbf{2NaOD}}{\text{ }} + {\text{ }}{{\mathbf{D}}_{\mathbf{2}}}$
3. Action on Metal Oxides:
When basic oxides like sodium monoxide and calcium oxide react with heavy water, they generate heavy alkalis.
${\mathbf{N}}{{\mathbf{a}}_{\mathbf{2}}}{\mathbf{O}}{\text{ }} + {\text{ }}{{\mathbf{D}}_{\mathbf{2}}}{\mathbf{O}}{\text{ }} \to {\text{ }}{\mathbf{2NaOD}}$
4. Action on Non-metal Oxides:
Deutero-acids are created when heavy water reacts with acidic non-metal oxides like sulphur trioxide, dinitrogen pentoxide, and others to form deutero-acids.
Hydrogen Peroxide:
Hydrogen peroxide is a very pale blue liquid that seems colourless. It is formed as a by-product of oxygen metabolism in living organisms. The hydrogen peroxide molecule is non-planar in structure. It's laid out like a book. In the gaseous and solid phases, the molecule has a distinct structure due to the existence of lone pairs of electrons on the oxygen atoms and hydrogen bonding. The dihedral angle of hydrogen peroxide in its gaseous form is ${111.5^ \circ }$
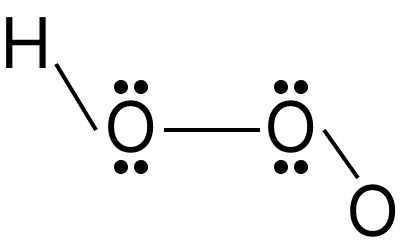
Hydrogen Peroxide
Preparation:
1. Laboratory Method:
Hydrogen peroxide is made in labs using barium peroxide or an acidified sulphate solution.
$Ba{O_2}.8{H_2}O\left( s \right){\text{ }} + {\text{ }}{H_2}S{O_4}\left( {aq} \right){\text{ }} \to {\text{ }}BaS{O_4}\left( s \right){\text{ }} + {\text{ }}{H_2}{O_2}\left( {aq} \right){\text{ }} + {\text{ }}8{H_2}O\left( I \right)$
2. Commercial Method:
The auto-oxidation of 2-ethylanthraquinol produces hydrogen peroxide. It entails the oxidation and reduction of 2-ethylanthraquinol in a cycle.
${\text{2 - ethyl anthraquinol}}\xrightarrow{{{{\text{O}}_{\text{2}}}{\text{/air}}}}{\text{2 - ethyl anthraquinone + hydrogen peroxide}}$
3. Electrolysis:
On electrolytic oxidation, hydrogen peroxide was also created from a 50 percent sulphuric acid solution. Hydrogen peroxide is generated at the anode during electrolysis. At the anode, peroxydisulphuric acid is produced as a by-product. At the cathode, hydrogen gas is produced.
$2HS{O^ - }_4\;\left( {aq} \right)\xrightarrow{{Electrolysis}}H{O_3}SOOS{O_3}H{\text{ }}\left( {aq} \right)\xrightarrow{{hydrolysis}}2HS{O^{\; - }}_4\;\left( {aq} \right){\text{ }} + {\text{ }}2{H^ + }\;\left( {aq} \right){\text{ }} + {\text{ }}{H_2}{O_2}\left( {aq} \right)$
Reactions:
Reaction in Acidic Medium:
It serves as an oxidizer, forming ferric ions when it interacts with ferrous ions.
$2F{e^{2 + }}\;\left( {aq} \right){\text{ }} + {\text{ }}2{H^ + }\;\left( {aq} \right){\text{ }} + {\text{ }}{H_2}{O_2}\;\left( {aq} \right){\text{ }} \to {\text{ }}2F{e^{3 + }}\;\left( {aq} \right){\text{ }} + {\text{ }}2{H_2}O\left( I \right)$
Reaction in Basic medium:
As an oxidizing agent, hydrogen peroxide reacts with ferric ions to form ferrous ions.
Uses:
In the textile and paper industries, hydrogen peroxide is utilised as a bleaching agent. According to data, about 60% of the world's Hydrogen Peroxide output is utilised for pulp and paper bleaching.
In everyday life, hydrogen peroxide may be used as a hair bleach as well as a mild disinfectant.
The manufacturing of sodium percarbonate and sodium perborate, which are used as mild bleaches in laundry detergents, is one of the most important industrial applications of this chemical.
Hydrogen peroxide has also been used to eliminate organic pollutants in some waste-water treatment systems.
Hydrogen peroxide is used to sterilise a variety of surfaces, including surgical instruments, and it may also be used to sterilise rooms as a vapour.
Hydrogen peroxide is a safer alternative to chlorine-based bleaches for the environment. It is also generally acknowledged as safe as an antibacterial agent.
Hydrogen Peroxide has been utilised for wound disinfection since the dawn of time, owing to its inexpensive cost and quick availability compared to other antiseptics. However, because it kills freshly created skin cells, it is now suspected to hinder healing and cause scarring.
Importance of Chemistry Hydrogen Notes
The chapter explains the element Hydrogen which is a part of the periodic table. It also describes some important characteristics of the element along with its physical properties. For instance, students can learn about the position of the Hydrogen element in the Periodic table. They will also learn the unique characteristics of Hydrogen and how it is different from other elements.
Some of the important subtopics such as the Hydrides and their different types, Dihydrogen, etc. are explained in detail. Further in the chapter, students will get to learn about the role of Hydrogen in the creation of water molecules. Since Hydrogen is an important element for the creation of water, students will learn the physical and chemical properties along with the structure of the molecule.
There are multiple other topics described in the chapter such as details about Heavy Water, Hydrogen Economy, Hydrogen Peroxide and so much more. This chapter properly describes to all the students how hydrogen can have different properties along with explanations of the uses of Hydrogen in real life. By studying the chapter, students will become completely familiar with the Hydrogen element. Download and refer to Class 11 Chemistry Chapter Hydrogen notes PDF right now for a detailed study session.
Benefits of Vedantu’s NEET Hydrogen Chapter Revision Notes
Students have all the help from the revision notes that have been provided for the chapter. The subject matter experts at Vedantu have carefully explained each and every single sub-topic of the chapter in a very concise manner for easy understanding of the chapter.
No longer do you have to worry about taking notes during class times because you can now download Hydrogen notes for NEET PDF easily from Vedantu. These files are easily accessible and can be used from any mobile device.
The revision notes for the chapter will help students revise all the different concepts and that too within a short period of time. With these notes, you can recall all the unique properties of hydrogen, its compounds, and much more.
Now you can find out exactly how the subject matter experts at Vedantu have answered all the important questions related to the chapter. Cross-check these answers to rectify your mistakes and clarify any doubts about the chapter.
Download Hydrogen Revision Notes PDF for NEET Preparation
For easy NEET preparation go for Hydrogen Class 11 Notes PDF download and find out the easiest ways to understand basic concepts related to the chapter. Download these notes and refer to them and figure out the important topics of the NEET examination. Anyone visiting the official website of Vedantu will have access to these files.
NEET Chemistry Revision Notes - Chapter Pages
NEET Chemistry Chapter-wise Revision Notes | |
Classification of Elements and Periodicity in Properties Notes | |
Hydrogen Notes | |
Other Important Links Related to NEET Hydrogen
Other Important Links for NEET Hydrogen |
FAQs on Revision Notes on Hydrogen for NEET 2026
1. What are some uses of Hydrogen?
Hydrogen is mainly used as rocket fuel due to the high calorific value that it has. Oxy Hydrogen Flame is used for different welding purposes. Also, Hydrogen is a completely reducing agent.
2. What are the physical properties of Hydrogen?
It is an odourless, colourless, and tasteless gas. The vapour density of Hydrogen is 1 and hence it is lighter than air. Hydrogen is not poisonous.
3. The combination of Dihydrogen with what gaseous compound results in the creation of Methanol?
The treatment of Dihydrogen with Carbon monoxide or CO results in the production of Methanol.
4. Mention the main components present in water gas?
Hydrogen and Carbon monoxide are present in water gas.




















 Watch Video
Watch Video




