Polymers NEET Questions: Elevate Your NEET Preparation for High Scores
Polymers is a very important chapter in Organic Chemistry that teaches students about different elements that exist in nature which are a combination of monomers. The chapter on Polymers has special significance since it is included in the syllabus for NEET Chemistry. Hence, students have to develop a thorough understanding of the chapter in order to tackle the difficult questions that are asked in this competitive exam. To aid this process, students can download and refer to Polymers important questions right now.
The important questions for the Polymers chapter have been created by the experts here at Vedantu and these are curated following the requirements and standard of the NEET entrance examination. Solving NEET Chemistry Polymers important questions will enable the students to know more about what polymers are and what properties, as well as uses, they have.
Access JEE NEET Important Questions Chemistry Polymers
Single Correct Type
1. Which of the following statements is not true about low-density polythene?
(A) Hard
(B) Tough
(C) Highly branched structure
(D) Poor conductor of electricity
2. Natural rubber has [NEET-2016]
(A) All cis-configuration
(B) All trans-configuration.
(C) Alternate cis-and trans-configuration.
(D) Random cis-and trans-configuration
3. Which of the monomers of neoprene in the following
(NEET 2003, 2013)
(a) $\mathrm{CH}_{2}=\mathrm{CH}-\mathrm{C}=\mathrm{CH}_{2}$
(b)
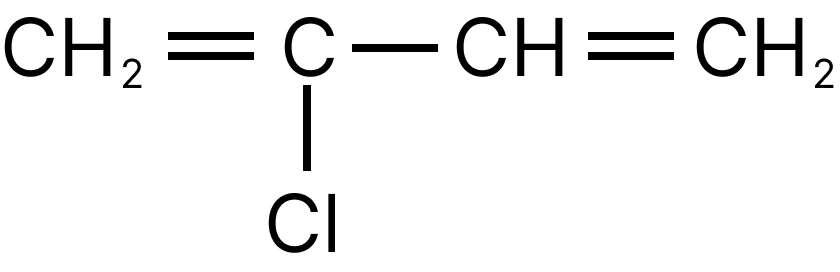
(c) $\mathrm{CH}_{2}=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}_{2}$
(d)
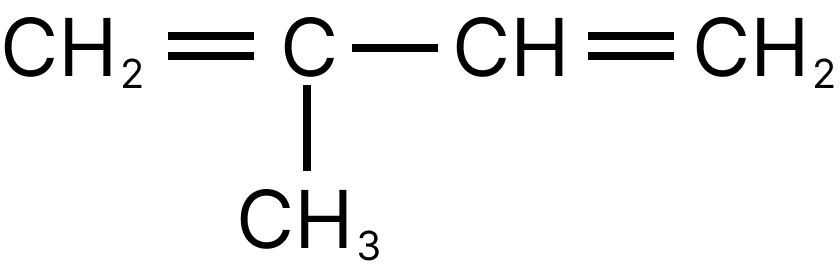
4. Arrange the following in ascending order of intramolecular force.
(a) Nylon-6, 6 (b) Buna-S (c) Polythene.
(A) a<b<c
(B) b<a<c
(C) b<c<a
(D) a=b=c
5. Natural rubber or gutta-percha
(A) Sis-polyisoprene and trans-polyisoprene
(B) Both are cis-polyisoprene
(C) Both are trans polyisoprene.
(D) Trans-polychloride and cis-polychloroprene
6. Soft drinks and baby bottles usually include
(A) Polyester
(B) Polyurethane
(C) Polystyrene
(D) Polyamide.
7. Non-stick cookware is generally coated with a polymer whose monomer is an organic compound.
(A) $C{H_2} = C{H_2}$
(B) $C{H_2} = CHCN$
(C) $C{H_2} = CHCl$
(D) $C{F_2} = C{F_2}$
8. What percentage of sulphur is used in vulcanized rubber?
(A) 5%
(B) 3%
(C) 55%
(D) 30%
9. Which of the following cannot be used as a monomer in the polymerization reaction?
(A) ${C_2}{H_4}$
(B) ${C_2}{H_2}$
(C) ${C_3}{H_6}$
(D) ${C_4}{H_6}$
10. Calculate the amount of polythene formed from 10 kg of calcium carbide from the reactions given below
$Ca{C_2} + 2{H_2}O \to Ca{\left( {OH} \right)_2} + {C_2}{H_2};$
$CH \equiv CH - {H_2}\xrightarrow{{pd - BaS{O_4}}}C{H_2} = C{H_2}$
$nC{H_2} = C{H_2} \to {\left( { - C{H_2} - C{H_2} - } \right)_n}$
(A) 28g
(B) 9g
(C) 4 kg
(D) 64 kg
11. Which of the following is the radical initiator.
(A) $R - N = N - R$
(B) Dibenzoyl peroxide.
(C)
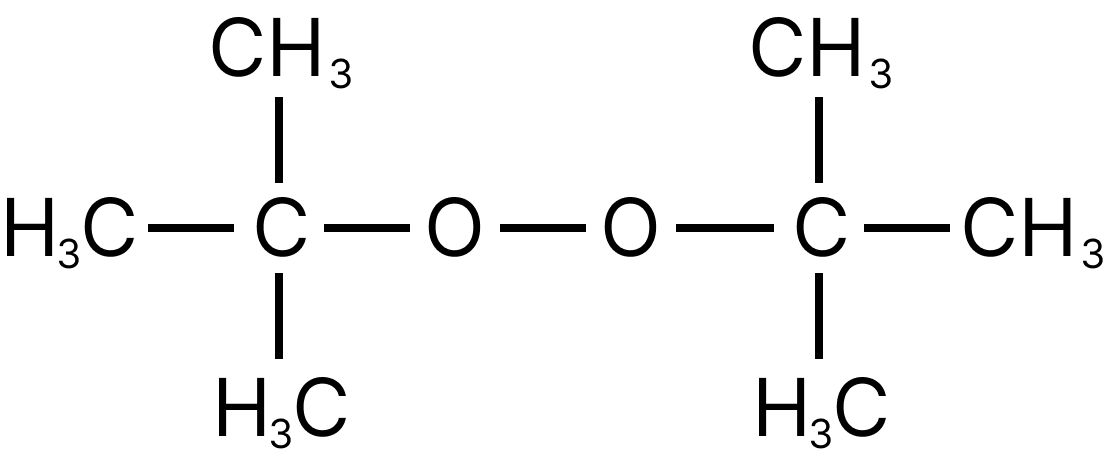
(D) All
12.
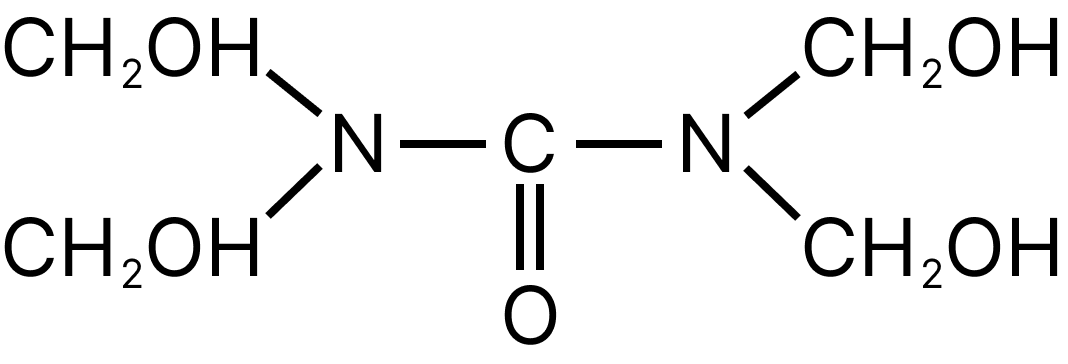
The polymer obtained by the above compound is:
(A) Bakelite
(B) Urea formaldehyde resin
(C) Melamine formaldehyde resin
(D) Teflon
13. Emphasize the most diverse polymerization forms of $C{H_2} = CH - CH = C{H_2}$
(A)
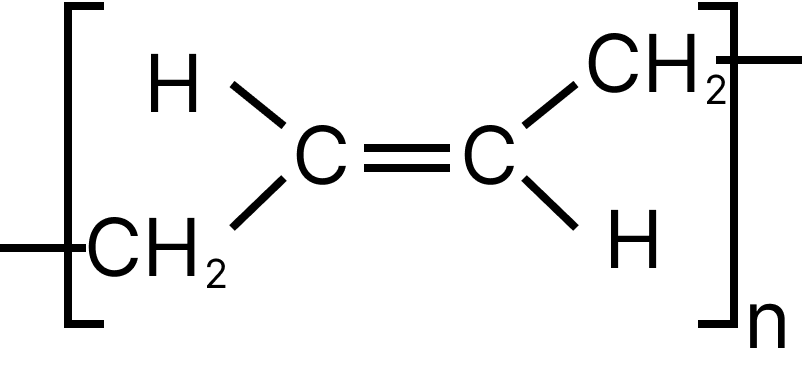
(B)
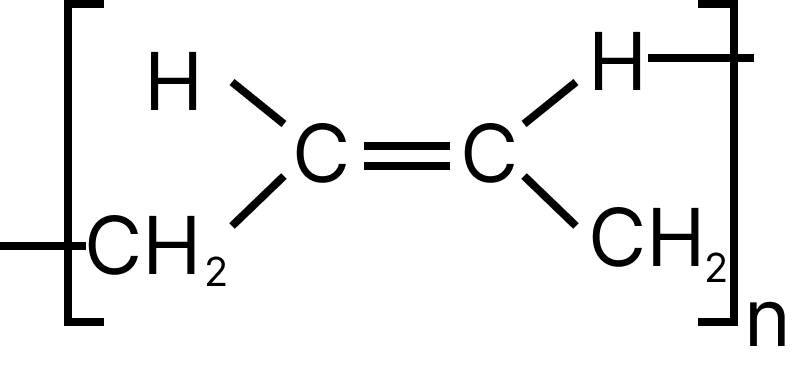
(C)
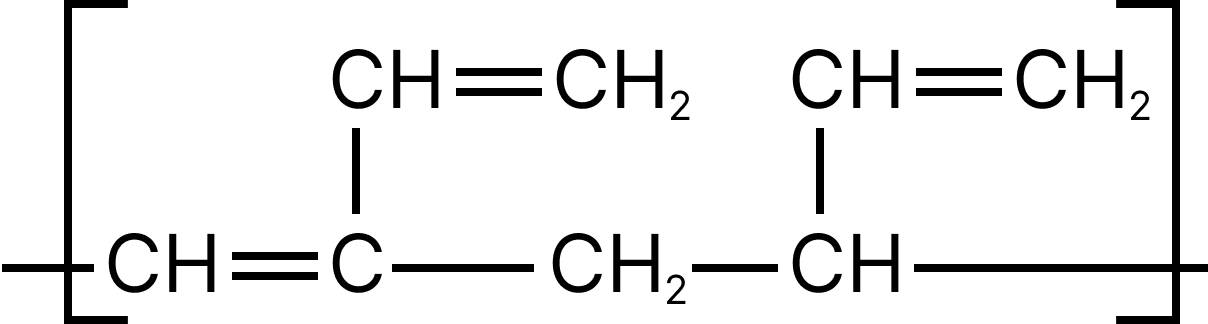
(D)
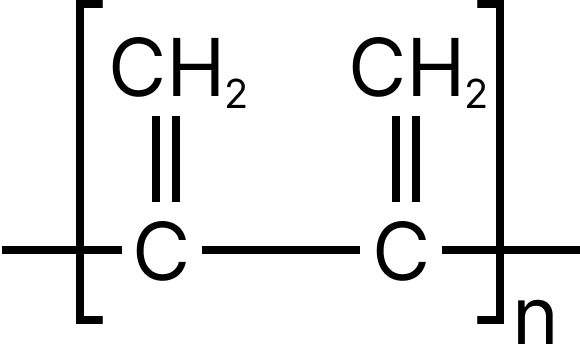
Multiple correct type
14. Which of the following monomers form biodegradable polymers? (NEET 2015)
(A) 3-hydroxybutanoic acid + 3-hydroxypentanoic acid
(B) Glycine + amino caproic acid
(C) Ethylene glycol + phthalic acid
(D) Caprolactam
15. Which of the following is a natural polymer? (NEET --2020)
A) poly (Butadiene-styrene)
B) polybutadiene
C) poly (Butadiene-acrylonitrile)
D) cis-1, 4-polyisoprene
16. Which of the following glucose polymers is preserved by the animal?
(A) Cellulose
(B) Amylose
(C) Amylopectin
(D) Glycogen.
17. When vulcanized, it becomes rubber
(A) More elastic
(B) Soluble in inorganic solvent
(C) Crystalline
(D) More stiff
18. Nylon-66 is made by using (AIIMS-1997)
(A) Succinic acid
(B) Benzyl chloride
(C) Benzaldehyde
(D) Adipic acid
19. Which of the following substances cannot be used as a plasticizer?
(A) Di-n-octylpythalate
(B) Di-n-butyl phthalate
(C) Tri cresol phosphate
(D) Sodium hexametaphosphate
20. Polymer used in bullet proof glass
(A) PMMA
(B) Lexan
(C) Nomex
(D) Kevlar
21. Chain-growth polymerization can proceed by the following mechanism.
(A) Condensation polymerization.
(B) Cationic polymerization.
(C) Anionic polymerization
(D) Free radical polymerization.
22. Which of the following is/are correct about polymers
(A) They are made by condensation of monomers in step growth polymerisation
(B) Nylon-66 is step-growth polymer
(C) Polystyrene is chain-growth polymer.
(D) The end of the chain is reactive because it is a radical, a cation or anion in chain polymerisation.
23. Examples of a biodegradable polymer pair is
(A) Nylon-6,6 and terylene.
(B) PHBV and dextron
(C) Bakelite and PVC
(D) PET and polyethylene
Assertion (A) and Reason (R) type Questions
1) Both (A) and (R) are true and (R) is the correct explanation of (A)
2) Both (A) and (R) are true and (R) is not the correct explanation of (A)
3) (A) is true but (R) is false
4) Both (A) and (R) are false.
24. (A): Glyptal is a condensation homopolymer
(R): Glyptal is used to prepare computer discs
25. (A): P.V.C is an addition copolymer
(R): P.V.C is used to prepare handles of utensils.
26. (A) Natural rubber is a polymer is isoprene.
(R): Isoprene is a pentene. (AIIMS-2014)
27. (A): Teflon is a chemically inert substance
(R): Chloroform when treated with antimony trifluoride gives Teflon. (AIIMS-2015)
28. (A): Polypropylene is an addition polymer
(R): Additional polymer is also named as step-growth polymer.
29. (A): PHBV is a homopolymer
(R): PHBV is a biodegradable polymer.
Solutions:
Single Correct Type
1. D
It's not difficult. It's hard, but it's mechanically weak. Due to its poor conductivity, it is used as an insulator for cables. It is a branched polymer. High density polyethylene is a linear polymer.
2. A
All-natural rubber is cis-arranged. A linear polymer of cis-isoprene or cis-2-methyl-1,3-butadiene is the chemical formula of rubber.
3. A
Chloroprene is the monomer of neoprene.
4. C
Different types of polymers have different intermolecular attractive forces. Elastomers or rubbers are the weakest and fibers have the strongest intermolecular attraction. Plastic has an intramolecular attractive force. Therefore, the ascending order of the intramolecular force of a given polymer is as follows: b<c<a
5. A
Natural rubber or gutta-percha is cis-polyisoprene and trans-polyisoprene. Isoprene is 2-methyl-1,3-butadiene. Natural rubber has long flexible chains, weak intramolecular force, and elastic. Gutta-percha has a zigzag chain that fits together. It is inelastic and crystalline.
6. C
Polystyrene is a synthetic thermoplastic polymer composed of styrene. It can be easily molded and is commonly used in protective packaging (such as peanut packaging), disposable cutlery, containers, bottles, trays, cups, baby bottles, and tissues.
7. D
Non-stick cookware usually has a polytetrafluoroethylene (PTFE) coating, a Teflon monomer
8. A
5% sulphur is used to increase the rigidity of the vulcanized rubber.
9. C
${C_3}{H_6}$ Is propylene, a monomer unit for the polymerization of polypropylene.
10. C
Molecular weight of $Ca{C_2} = 40 + 24 = 64$
64 kg of $Ca{C_2}$ gives 28 kg of ethene
10 kg of $Ca{C_2}$ gives $\dfrac{{28}}{{64}} \times 10 = \dfrac{{7 \times 10}}{{16}} = 4.37kg$
11. D
All are free-radical initiators and catalyze the free radical polymerisation
12. B
The given polymer is called urea-formaldehyde resin because it is made up of urea and formaldehyde.
13. D
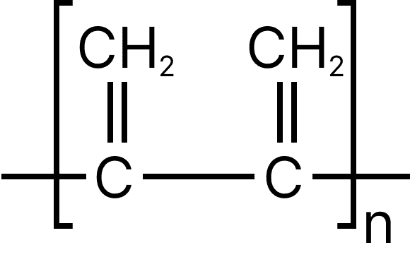
Both the double bonds are preserved i.e., they are not involved in polymerization, hence it is most unlike form of polymerization.
14. A, B
Polymers that are easily decomposed are known as biodegradable polymers. PHBV is biodegradable in nature. It is formed by the copolymerization of 3-hydroxybutyric acid and 3-hydroxypentanoic acid. Glycine + aminocaproic acid copolymerize to form nylon-2-nylon 6. This is also biodegradable
15. B, C
Buna-S and neoprene require at least one diene monomer at the time of manufacture. The copolymerization of 1,3-butadiene and styrene produces Buna-S in the presence of a peroxide catalyst. The free radical polymerisation of chloroprene forms neoprene.
16. D
Animals store glycogen
Carbohydrates are stored in animals as glycogen. It has a structure similar to that of amylopectin and is slightly branched, so it is also called animal starch. It is present in the liver, muscles and brain.
17. A, D
Vulcanization is a chemical process in which rubber is heated with sulphur to form crosslinks between rubber molecules. This process is performed to improve the physical properties such as elasticity of natural rubber. It makes the rubber stiffer.
18. C
Nylon 66 is a type of polyamide or nylon. Nylon 66 and Nylon 6 are the two most commonly used elements in the textile and plastics industry. Nylon 66 is made up of two monomers, each containing six carbon atoms, hexamethylenediamine and adipic acid, and is named after Nylon 66.
19. B, C
20. B
Lexan is a type of polycarbonate. H. Thermosetting polymer containing carbonic acid groups. They are strong, durable materials and are optically transparent. For this reason, laminated polycarbonate (Lexan) is used to manufacture bullet proof glass.
21. B
Condensation polymers are formed by a condensation reaction in which molecules bind and loose small molecules as a by-product. For example, nylon 6, 6 is formed by condensation polymerization of adipic acid and hexamethylenediamine. Polystyrene, Teflon, and rubber, on the other hand, are all formed by chain-growth polymerization of the monomer’s styrene, tetrafluoroethylene, and isoprene, respectively.
Here, unsaturated monomer molecules are added individually to the active site of the growing polymer chain.
22. C
TEP growth polymerization refers to a type of polymerization mechanism in which bifunctional or multifunctional monomers react to form a dimer first, then a trimer, a longer oligomer, and finally a long-chain polymer.
For example, nylon is polymerized by a step-by-step growth polymerization process in which bifunctional monomers containing equal amounts of amines and carboxylic acids react to form amides at both ends of each monomer.
Polyacrylonitrile, polyisoprene, and polyethylene are polymerized by chain-growth polymerization of acrylonitrile, isoprene (2-methyl-1,3-butadiene), and ethane, respectively.
Chain-growth polymerization (or addition polymerization) involves the bonding of molecules, including double or triple carbon-carbon bonds.
23. B
Biodegradability is defined as the ability to be degraded by other organisms. Nylon-2-Nylon-6, Dextran, and PHBV are biodegradable, but Nylon-6,6 is not.
However, both dextran and PHBV are polyester, and nylon-2-nylon-6 is polyamide. The polymerization of many sugar molecules forms dextran.
24. D
Glyptal is a copolymer of ethylene glycol and phthalic acid. Bakelite is a condensed polymer (copolymer) of phenol and formaldehyde.
25. D
Polyvinyl chloride (PVCA) is a thermoplastic copolymer of vinyl chloride and acetate.
26. C
Isoprene is 2-methyl-1, 3-butadiene.
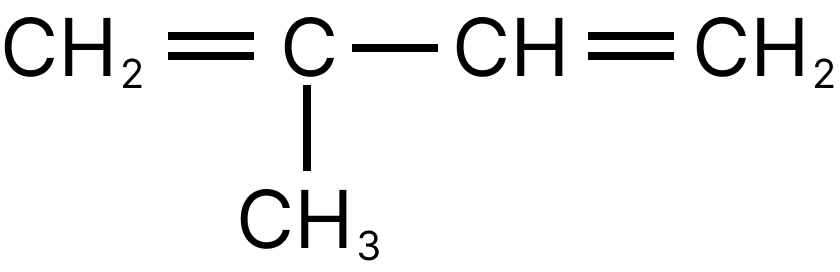
27. C
Treatment of chloroform with antimony trifluoride and hydrofluoric acid produces tetrafluoroethylene. Upon polymerization, tetrafluoroethylene forms Teflon.
28. A
Polypropylene is an example of an additive polymer. b. Additional polymers result from repeated additions of monomer units. The monomers involved are unsaturated compounds, usual derivatives of ethene. Example: Propylene undergoes additional polymerization to form polypropylene. In addition to polymerization, monomeric units are continuously added to the growing chain. By chain reaction, it is called chain-growth polymerization
29. A
The copolymer is PHBV. Poly (hydroxybutyrate-covariate) (PHBV) is a fully biodegradable thermoplastic polyester produced by microbial fermentation.
Importance of NEET Chemistry Polymers Chapter for NEET Preparation
NEET students have to prepare the chapter on Polymers as it is a part of their syllabus for the medical competitive examination. Polymers can be defined as compounds that are created with the combination of one or more types of monomers. Some of the main examples of polymers include proteins and polysaccharides. Students need to read the chapter on Polymers carefully in order to understand the contents and answer the questions related to the topics.
Students will have to study Polymers important topics to understand what polymers are in detail and how they can be created in nature. They will learn crucial concepts such as natural and synthetic polymers while familiarising themselves with the theory of synthesising polymers. They need to be aware of the different concepts related to the synthesis of polymers. They will learn about addition and condensation synthesising, which is responsible for the creation of polymers.
The chapter also teaches students about the different mechanisms related to the process of polymerization like Chain Growth and Step Growth Polymerization. Students are also introduced to the topic of copolymerisation, which is the process that includes the polymerization of two different types of polymers. They can learn different examples of polymers such as Polythene, Nylon, and others while understanding the process through which these polymers are formed.
By practising from Polymers Class 12 important questions and answers PDF, students will be able to grasp the contents of the chapter. These questions have detailed answers in them which can aid in the revision of the chapter as well.
Benefits of Important Questions for Polymers Chapter from Vedantu
Students will be able to grasp different concepts related to the chapter from the important questions. Solving these questions will give them enough practice to go ahead and tackle the more challenging questions of NEET.
Experts at Vedantu have carefully crafted all the questions from the chapter to give the students a concise idea about what the chapter is. Every single question in the PDF files is explained methodically in order to make sure that students have no trouble understanding.
Preparing for NEET by solving the important questions related to these essential concepts will enable students to gain more confidence in the process. They can also clarify their doubts about certain questions and learn from experts how to solve different problems.
Studying the Polymers important questions NEET offered by experts at Vedantu, students can easily figure out the pattern of questioning used in NEET. They can also figure out which topics are important for the chapter and dedicate their attention to those.
Get Polymers Class 12 Important Questions for NEET Preparation Now
Download Polymers important questions PDF from Vedantu and include these in your study materials today. The questions have been framed by the subject experts at Vedantu in adherence to the NEET standards and high-score requirements. Hence, the free PDF file will provide you with the chance to score high marks in the NEET examination.
FAQs on NEET Important Questions for Class 12 Chemistry Polymers
1. Define copolymerization.
Copolymerization is a type of polymerization that takes place when there are two different types of monomers combining together to form a single polymer which is called a copolymer.
2. What is additional polymerization?
Addition polymerization describes the process where the monomers are adding successively to an existing polymer chain that has a reactive intermediate species such as a free radical or anion.
3. Provide an example of elastomers.
Buna-N and Buna-S are some examples of elastomers.
4. Mention the use of Bakelite.
Bakelite is a major polymer that is used for the creation of utensil handles. It is also a primary product for creating pieces of dominoes and chess.

























