




Introduction to Solutions
We rarely come up with pure substances in everyday life. The majority of them are having blends of two or more pure chemicals. Their usefulness or significance in life is determined on the basis of their composition For instance, Like Brass (a copper-zinc alloy) is a unique material from those made of German silver (copper, zinc, and other metals and nickel) or bronze (copper and tin combination); Fluoride ions in water at 1 part per million (ppm) inhibits tooth decay, whereas 1.5 ppm results in tooth decay may become mottled, and high fluoride concentrations. Ions can be harmful (sodium fluoride, for example). Intravenous injections are always utilised in rat poison dissolved in water containing salts with certain ionic properties concentrations that are similar to those found in blood plasma.
Important Topics for Solutions
Introduction to Solutions
Expressing Concentration of Solutions
Vapour Pressure of Liquid Solutions
Types of Solutions
Solubility
Ideal and Non-ideal Solutions
Colligative Properties and Determination of Molar Mass
Abnormal Molar Masses
1. Types of Solutions
The term "solution" refers to a homogenous mixture of two or more substance components. The term "homogeneous mixture" refers to a mixture whose composition and attributes are consistent throughout. Solvent refers to the component that is present in the greatest quantity. The physical condition of a solution is determined by the solvent. Solutes are one or more components in a solution that are not the solvent.
2. Expressing Concentration of Solutions
There are various quantitative methods for describing the concentration of a solution.
(a) (w/w) Mass Percentage:
Mass percentage in a solution is defined as
Mass % of a component = (Mass of the component in the solution / Total mass of the solution) x 100
(b) Volume Percentage (V/V):
The volume percentage is described as
Volume % of a component = (Volume of the component/ Total volume of solution) x 100
(c) Mass by Volume Percentage (w/V):
Another unit which is mostly used in medicine and pharmacy is mass by volume percentage. It is defined as the mass of solute dissolved in 100 mL of the solution.
(d) Parts Per Million:
When a solute is present in trace quantities, it is convenient to express concentration in parts per million (ppm) which is defined as
Parts per million = (Number of parts of the component/Total number of parts of all components of the solution) x 106
(e) Mole Fraction:
Most Commonly used symbol for mole fraction is x and the subscript used on the right-hand side of x denotes the component. It is defined as - Mole fraction of a component = number of moles of the component / Total number of moles of all the components.
(f) Molarity:
It is defined as the number of moles of solute dissolved in one litre (or one cubic decimetre) of solution.
M= Moles of solute Molarity / Volume of solution in a litre
(g) Molality:
It is defined as the number of moles of the solute per kilogram (kg) of the solvent and is expressed as:
Molality (m) = Moles of solute / Mass of solvent in kg
3. Solubility
A substance's solubility refers to the greatest amount that may be dissolved in a given amount of solvent at a given temperature. The type of the solute and solvent, as well as their temperature and pressure, play a very important role.
(a) Solubility of a Solid in a Liquid
When a solid solute is dissolved in a solvent, some of the solutes dissolve and the concentration of the solution increases. Dissolution is the term for this procedure. Some solution solute particles collide with solid solute particles, causing them to detach from the solution. Crystallisation is the name for this process. When the two processes occur at the same time, a stage is reached. Under these conditions, the number of solute particles entering the solution equals the number of solute particles separating out, resulting in a state of dynamic equilibrium.
(b) Solubility of a Gas in a Liquid
Water dissolves a lot of gases. Only a small amount of oxygen dissolves in water. All aquatic life relies on dissolved oxygen to survive. Hydrogen chloride gas (HCl), on the other hand, is very soluble in water.
Pressure and temperature have a significant impact on the solubility of gases in liquids. The solubility of gases increases as pressure rises.
Henry established Henry's law, which is a quantitative relationship between pressure and solubility of a gas in a solvent. The law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas existing above the liquid or solution's surface at a constant temperature.
The partial pressure of the gas above the solution is proportional to the mole fraction of gas in the solution. Henry's law states that "the partial pressure of the gas in the vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution" and is written as-
p = KH x
The KH stands for Henry's law constant.
Henry's law has a number of industrial applications and also explains several biological occurrences. The bottle is sealed under high pressure to improve the solubility of CO2 in soft drinks and soda water, and scuba divers must deal with large amounts of dissolved gases while breathing air at high pressure underwater. The solubility of ambient gases in blood increases as pressure rises.
The pressure progressively lessens as the divers approach the surface. This causes the dissolved gases to be released, resulting in the development of nitrogen bubbles in the blood. This causes capillaries to constrict, resulting in bends, a painful and life-threatening medical condition.
In order to avoid bends and the harmful effects of high concentrations,
Scuba divers' tanks are loaded with nitrogen to compensate for the nitrogen in their blood. Using helium-dilution air (11.7 percent helium, 56.2 percent nitrogen, and (32.1%) of oxygen).
The partial pressure of oxygen at high altitudes is lower than at ground level. Low oxygen concentrations at high altitude areas result in low amounts of oxygen in blood cells and tissues of a person who lives at high elevations or mountain climbers. Climbers become weak and unable to think as a result of a lack of oxygen in their blood. Clearly, the signs and symptoms of anoxia are seen.
4. Vapour Pressure of Liquid Solutions
When the solvent is a liquid, liquid solutions are created. A gas, a liquid, or a solid can be the solute. The solutions of liquids and solids in a liquid will be discussed in this section. One or more volatile components may be present in such solutions. The liquid solvent is usually volatile.
The solute could be volatile or not. We will exclusively address the properties of binary solutions, or solutions with two components, such as solutions of -
(i) Liquids in Liquids
(ii) Solids in Liquids
(a) Vapour Pressure of Liquid Solutions-
Francois Marte Raoult (1886), a French chemist, established the quantitative link between them. The relationship is known as Raoult's law, which asserts that for a solution of volatile liquids, the Vapour Pressure of Liquid Solutions is given as-
p1 = p01 x1
where p01 is the vapour pressure of pure component 1 at the same temperature.
Similarly, for component 2
p2 = p02 x2
ptotal = p1 + p2
The above statement is according to the dalton's law of partial pressure -
Substituting the values of p1 and p2 , we get
ptotal = p01 x1 + p02 x2
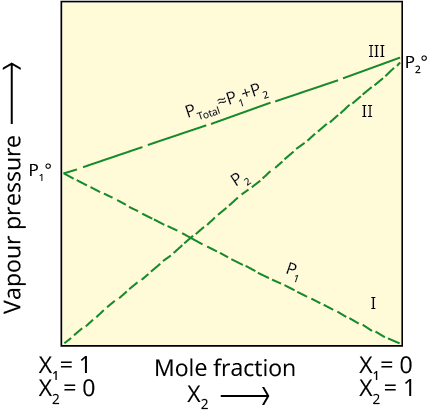
(b) Vapour Pressure of Solutions of Solids in Liquids
The solution's vapour pressure at a given temperature is found to be lower than the liquid's vapour pressure. At the same temperature, use a pure solvent. In both the solute and the surface of the solution molecule of the solvent; as a result, the percentage of the solvent molecules covering the surface decreases. As a result, the number of molecules of the solvent gets away from the surface. As a result, the vapour pressure is lowered. The amount of pressure is also lowered. The drop in solvent vapour pressure is determined by the amount of non-volatile solute present in the solution.
5. Ideal and Non-ideal Solutions
(a) Ideal Solutions
An ideal solution is one in which each component follows Raoult's rule under all temperature and concentration circumstances.
Ideal solutions have the following characteristics:
Change in enthalpy = 0; Change in volume = 0;
The attractive forces between the A-A and B-B molecules are approximately comparable to those between the A-B molecules.
For Example: benzene and toluene solution, n-hexane and n-heptane solution.
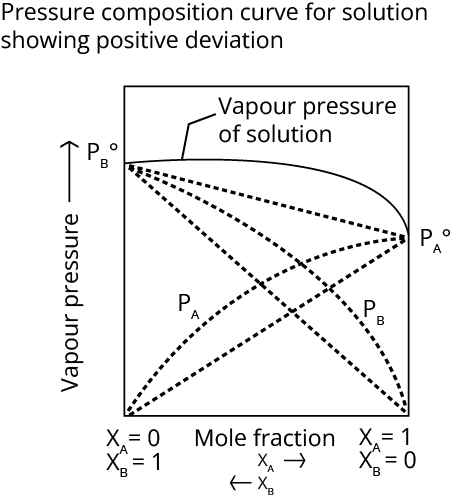
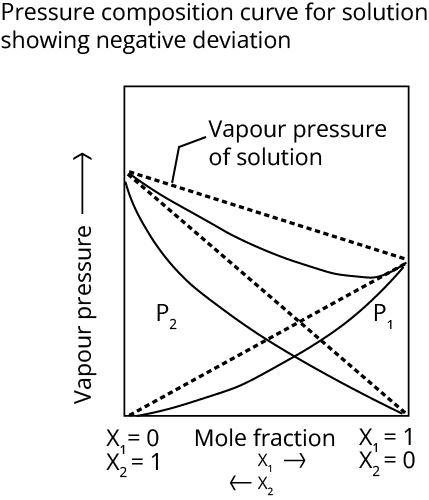
(b) Non-Ideal Solutions
The term "non-ideal solution" refers to a solution that does not obey Raoult's law over the whole concentration range. Non-ideal solutions show two types of deviations- negative deviation and positive deviation.
The Solvent-Solute(A-B) force is less powerful than the Solute-Solute(B-B) and Solvent-Solute (A-A) forces.
The vapour pressure is higher than the law predicts.
Change in enthalpy >0; Change in volume >0;
Examples- Ethanol with acetone, and carbon disulphide and acetone.
(c) Azeotropes
Azeotropes are binary mixes that boil at the same temperature and have the same composition in the liquid and vapour phases.
Azeotrope azeotrope azeotrope azeotrope a
Minimum boiling azeotrope at a certain composition is formed by solutions that demonstrate a considerable positive departure from Raoult's law.
Maximum boiling azeotrope at a certain composition is formed by solutions that demonstrate a substantial negative divergence from Raoult's law. A mixture of 68 percent nitric acid and water produces an azeotrope with a boiling point of 393.5 K.
6. Colligative Properties and Determination of Molar Mass
Colligative qualities are those that are dependent on the number of solute particles present in a solution, regardless of their type relative to the total number of particles present.
There are four colligative properties:
i. Relative Lowering of Vapour Pressure
ii. Elevation in Boiling Point
iii. Depression in freezing point
iv. Osmotic pressure
These can be described as -
i. Relative Lowering of Vapour Pressure
Relative lowering of vapour pressure is equal to the mole fraction of the solute. The above equation can be written as:
p01-p1/p1 = n2/n1+n2
Here n1 and n2 are the number of moles of solvent and solute respectively present in the solution.
ii. Elevation in Boiling Point
The temperature at which a liquid's vapour pressure equals atmospheric pressure is known as the boiling point of the liquid.
The vapour pressure of the solvent decreases when a non-volatile solute is added, hence the needed temperature to boil the solution will be higher. As a result, the boiling point of the solution will rise. The mathematical expression is given as-
ΔTb=Kb.m
Where Kb is called Boiling Point Elevation Constant or Molal Elevation Constant (Ebullioscopic Constant).
m is the molality.
ΔTb is the elevation in boiling point.
iii. Depression in Freezing Point
The freezing point of a substance is defined as the temperature at which the vapour pressure of the substance in its liquid phase is equal to its vapour pressure in the
solid phase.
The mathematical expression is given as-
ΔTf=Kf.m
Kf is known as Freezing Point Depression Constant or Molal Depression Constant or Cryoscopic Constant.
m is the molality.
ΔTf is the elevation in boiling point.
iv. Osmosis
When a pure solvent and solution are separated by a semipermeable membrane, the solvent remains pure. Particles go from the solvent to the solution side of the membrane. "Osmosis" is the name for this phenomenon. The semipermeable membrane allows only small molecules to pass through while blocking bigger solute molecules.
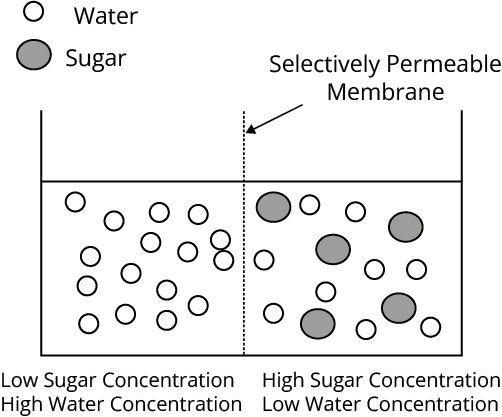
Osmotic Pressure
The extra pressure that must be provided to a solution to prevent osmosis, i.e., to stop the passage of solvent molecules through a semipermeable barrier into the solution, is known as osmotic pressure.
For dilute solutions, osmotic pressure is proportional to the molarity, C of the solution at a given temperature T.
Therefore,
π = CRT
Here π is the osmotic pressure and R is the gas constant.
Isotonic solutions are two liquids that have the same osmotic pressure at the same temperature.
In comparison to a more concentrated solution, a solution with a lower concentration or lower osmotic pressure is referred to as "Hypotonic."
In comparison to a dilute solution, a solution with a higher concentration or higher osmotic pressure is referred to as "hypertonic."
Reverse Osmosis
It occurs when a pressure greater than the osmotic pressure is applied to the solution side of the membrane, allowing the solvent to flow from the solution into the pure solvent. The process is known as reverse osmosis.
Applicability:
Desalination of seawater: When a pressure greater than the osmotic pressure is applied to the membrane, pure water is squeezed out of the seawater.
7. Abnormal Molar Masses
The molecular mass of a substance is said to have aberrant molar mass when it is determined by investigating any of the colligative qualities and differs from the theoretically expected value.
Molar masses that are abnormal are observed:
1. When the solute in the solution forms a connection.
2. When the solute in the solution undergoes dissociation.
The Van't Hoff Factor was created by Van't Hoff to calculate the level of association or dissociation. The mathematical expression can be given as-
i = normal molar mass / Abnormal Molar mass
i = colligative property observed /colligative property calculated
i = After association, the total number of moles of particles is (dissociation) / Before association, the number of moles of particles was counted (dissociation)
Solved Examples from The Chapter
Example 1. When chloroform and acetone are mixed, why does the temperature rise?
Solution: The bonds between chloroform molecules and other molecules are broken. There are dipole-dipole interactions in acetone, but when it is mixed, there are no dipole-dipole interactions. The molecules of chloroform and acetone begin to form hydrogen bonds that are stronger than other types of bonds, resulting in the act of releasing energy. As a result, there is an increase in temperature.
Key points to remember - Need to remember the ideal and non-ideal concepts and deviations from ideal solutions.
Example 2. Why does a mixture of ethanol and cyclohexane deviate from Raoult's law in a favourable way?
Solution: When cyclohexane is added, its molecules get stuck between the ethanol molecules, disrupting hydrogen bonds and lowering ethanol-ethanol interactions. This will raise the solution's vapour pressure, causing a positive divergence from Raoult's law.
Key points to remember - Force of attraction occurs in the deviation from Raoult's law.
Solved Questions from The Previous Year Question Papers
Question 1:The mixture which shows positive deviation from Raoult’s law is :
(a) Acetone + Chloroform
(b) Chloroethane + Bromoethane
(c) Ethanol + Acetone
(d) Benzene + Toluene
Solution: In positive deviation from Raoult's Law, the resulting solution has more vapour pressure than the ideal solution of the constituent liquids. Because the A-B interactions are weaker than A-A & B-B ones. As there is hydrogen bonding present in the ethyl alcohol solution and acetone molecules tend to occupy the space between them due to which some hydrogen bonds break, resulting in the weakening of A−B interaction.
Therefore, option (c) is the correct answer.
Trick: Need to remember the deviations from the ideal solutions that are positive and negative deviations from Raoult's law.
Question 2: If the molality of the dilute solution is doubled, the value of molal depression constant (Kf) will be
(a) Halved
(b) Tripled
(c) Unchanged
(d) Doubled
Solution: The value of molal depression constant, Kf is constant for a particular solvent, therefore, it will be unchanged when molality of the dilute solution is doubled.
Therefore, option (c) is the answer.
Trick: The concept used here is that depression in freezing point and molal depression have no effect on diluting a solution.
Question 3: A solution of two liquids boils at a temperature more than the boiling point of either of them. Hence, the binary solution shows
(a) Negative deviation from Raoult’s law
(b) Positive deviation from Raoult’s law
(c) No deviation from Raoult’s law
(d) Positive or negative deviation from
Solution: Solutions boil at a higher temperature, the vapour pressure of the solution is less. Hence, it shows a negative deviation from Raoult's law.
Therefore, option (a) is the correct answer.
Trick: Need to remember the deviations from the ideal solutions that are positive and negative deviations from Raoult's law.
Practice Questions
Question 1:The Van’t Hoff factors i for an electrolyte which undergoes dissociation and association in solvents are respectively
(a) Greater than 1 and greater than 1
(b) Less than 1 and greater than 1
(c) Less than 1 and less than 1
(d) Greater than 1 and less than 1
Answer: (d) Greater than 1 and less than 1.
Question 2: What happens when an egg is kept in a saturated
solution of NaCl after removing its hard shell
in dil. HCl?
(a) Egg will swell
(b) Egg will shrink
(c) Egg will remain same
(d) Egg will first shrink and then swell.
Answer: (b) Egg will shrink.
Question 3: Maximum freezing point falls in
(a) Camphor
(b) Naphthalene
(c) Benzene
(d) Water
Answer: (a) Camphor
Conclusion
A solution is a mixture of two or more components that is homogenous. Solid, liquid, and gaseous solutions are the three types of solutions. A solution's concentration is measured in mole fractions, molarity, molality, and percentages. Henry's law governs the dissolution of a gas in a liquid, which states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas at a particular temperature. Colligative qualities are those of solutions that are dependent on the amount of solute particles but not on their chemical identity. Vapour pressure is reduced, the boiling point is raised, the freezing point is lowered, and osmotic pressure is reduced. If a larger pressure is applied, the osmosis process can be reversed.
This reduces negative effects and concentrates the drug's efficacy. Drug chemistry is concerned with stopping or killing germs, keeping the body from contracting numerous infectious illnesses, and relieving mental stress, among other things.
NEET Chapter - Solutions

 Share
ShareFAQs on NEET Chapter - Solutions
1. What are the three most important characteristics of a solution?
The following are the properties of a solution:
A homogenous mixture is a solution. A homogeneous mixture is one in which two or more substances are present. The solution is invisible to the naked eye. Because the particles are thoroughly mixed together. A solution prevents light beams from scattering.
2. What is a solution's most active component?
The solvent is the component with the highest concentration, whereas the solute is the component with the lowest concentration. A physical process, not a chemical one, results in the production of a solution from a solute and a solvent. When miscible substances, such as gases, are mixed, they create a single-phase in all amounts.
3. What is the significance of a solution?
Any of the phases of matter can be dissolved in another phase of a solution, which is a homogenous mixture of molecules. Whether it's solids, liquids, or gases, solution chemistry is significant since most chemical reactions take place in solutions, whether in the lab or in nature.




















 Watch Video
Watch Video


