
$1$-phenyl ethanol can be prepared by the reaction of benzaldehyde with?
A. Methyl bromide
B. Ethyl iodide and magnesium
C. Methyl iodide and magnesium
D. Methyl bromide and aluminium bromide
Answer
577.8k+ views
Hint: Keep in mind that, when a carbonyl (i.e. an aldehyde or ketone) is treated with the Grignard reagent, the reactants will undergo Grignard reaction and will produce a secondary or tertiary alcohol. So, here benzaldehyde is given which is an aldehyde.
Complete step by step solution:
Given that,
$1$-phenyl ethanol can be prepared by the reaction of benzaldehyde.
Here, benzaldehyde is a carbonyl i.e. it is an aldehyde and has a chemical formula, ${{C}_{6}}{{H}_{5}}CHO$ while $1$-phenyl ethanol is an alcohol and has chemical formula, ${{C}_{6}}{{H}_{5}}C{{H}_{2}}(OH)C{{H}_{3}}$. Thus, we can say that the reactant is benzaldehyde and the product is $1$-phenyl ethanol.
So, we should have knowledge that whenever a carbonyl is given in the reactant and in product alcohol is formed, Grignard reagent ($RMgX$) must be used, where R refers to the alkyl group and X refers to a halogen. As benzaldehyde is given and $1$-phenyl ethanol is formed, the alkyl group used will have one carbon in its structure.
So, here methyl bromide when treated with benzaldehyde cannot form alcohol as it is not a Grignard reagent and there is an absence of highly electropositive magnesium which generally makes methyl group negative. But here the methyl group is positive and it cannot attack the carbonyl carbon.
Moving forward to ethyl iodide and magnesium, ethyl iodide is a two-carbon containing alkyl group. So, it will not form $1$-phenyl ethanol.
In case of methyl iodide and magnesium, it is a one-carbon containing alkyl group and it has magnesium in it which will make the methyl group negative. This negative methyl group will attack on the positive carbon present in the carbonyl group and will form an alcohol. The following chemical reaction is shown:
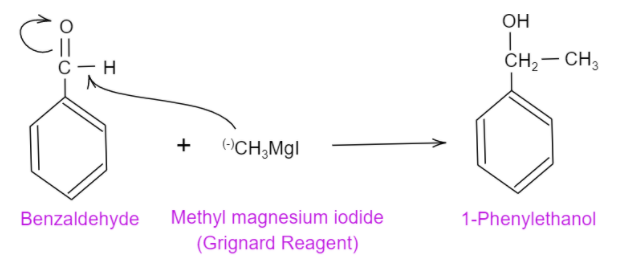
In case of methyl bromide and aluminium bromide, the aluminium bromide will attract the bromine towards itself making the methyl group positive. So, here also the positive methyl group will not attack the positive carbon on the carbonyl group and will not yield alcohol.
Hence, the correct option is C.
Note: Possibly you may get confused with the option B, where ethyl iodide and magnesium are given. It is important to note that ethyl iodide has a two-carbon alkyl group. Thus, it will yield propanol when reacted with benzaldehyde instead of ethanol.
Complete step by step solution:
Given that,
$1$-phenyl ethanol can be prepared by the reaction of benzaldehyde.
Here, benzaldehyde is a carbonyl i.e. it is an aldehyde and has a chemical formula, ${{C}_{6}}{{H}_{5}}CHO$ while $1$-phenyl ethanol is an alcohol and has chemical formula, ${{C}_{6}}{{H}_{5}}C{{H}_{2}}(OH)C{{H}_{3}}$. Thus, we can say that the reactant is benzaldehyde and the product is $1$-phenyl ethanol.
So, we should have knowledge that whenever a carbonyl is given in the reactant and in product alcohol is formed, Grignard reagent ($RMgX$) must be used, where R refers to the alkyl group and X refers to a halogen. As benzaldehyde is given and $1$-phenyl ethanol is formed, the alkyl group used will have one carbon in its structure.
So, here methyl bromide when treated with benzaldehyde cannot form alcohol as it is not a Grignard reagent and there is an absence of highly electropositive magnesium which generally makes methyl group negative. But here the methyl group is positive and it cannot attack the carbonyl carbon.
Moving forward to ethyl iodide and magnesium, ethyl iodide is a two-carbon containing alkyl group. So, it will not form $1$-phenyl ethanol.
In case of methyl iodide and magnesium, it is a one-carbon containing alkyl group and it has magnesium in it which will make the methyl group negative. This negative methyl group will attack on the positive carbon present in the carbonyl group and will form an alcohol. The following chemical reaction is shown:

In case of methyl bromide and aluminium bromide, the aluminium bromide will attract the bromine towards itself making the methyl group positive. So, here also the positive methyl group will not attack the positive carbon on the carbonyl group and will not yield alcohol.
Hence, the correct option is C.
Note: Possibly you may get confused with the option B, where ethyl iodide and magnesium are given. It is important to note that ethyl iodide has a two-carbon alkyl group. Thus, it will yield propanol when reacted with benzaldehyde instead of ethanol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




