
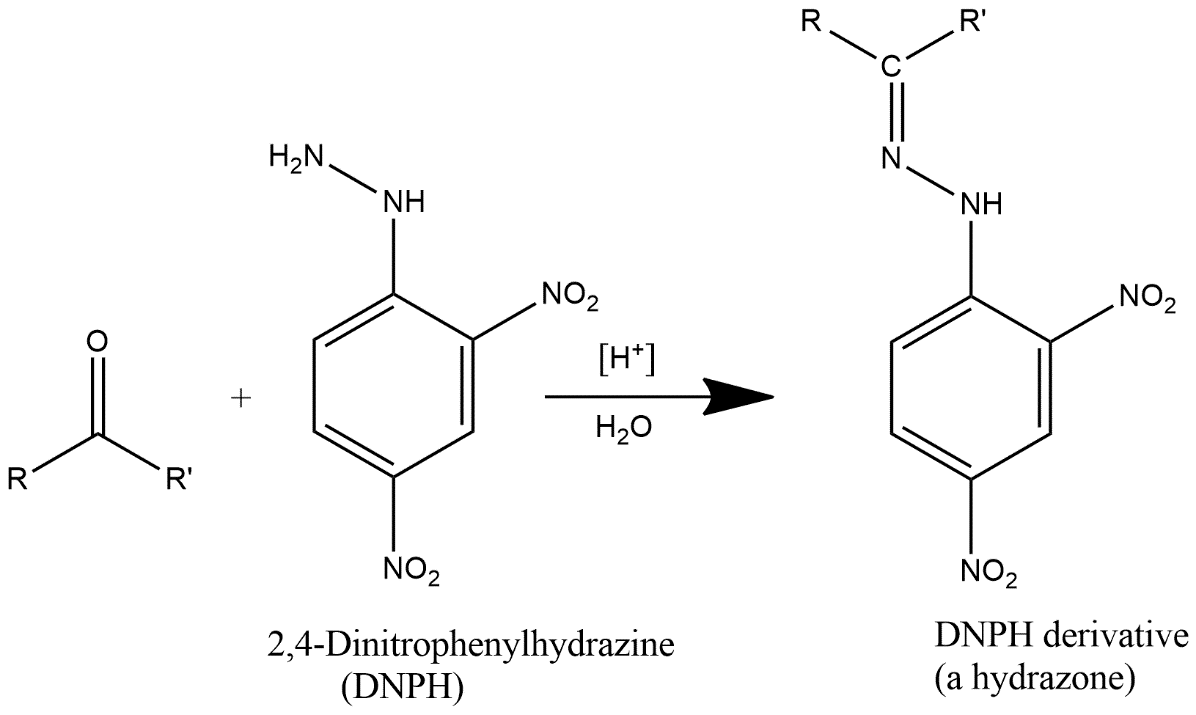
2,4 DNP reacts with carbonyls (aldehydes/ketones) to give yellow ppt. This reaction is a dehydration type reaction as shown above.
A mixture of benzaldehyde and formaldehyde is heated with 2,4DNP. How many organic products will be obtained after fractional distillation?

Answer
573k+ views
2,4-DNPH is generally known by the name 2,4-Dinitrophenylhydrazine also known by Brady’s or Borche’s reagent. It is represented by the chemical formula ${{C}_{6}}{{H}_{3}}{{(N{{O}_{2}})}_{2}}NHN{{H}_{2}}$. It is a substitute for hydrazine and red to orange solid.
Complete step by step answer: DNP is generally used as a test to identify the carbonyl group which is associated with aldehyde or ketone. It is generally available as a wet powder which is prepared by the reaction of hydrazine sulfate with 2,4-dinitrochlorobenzene. It is prepared by dissolving 2,4-dinitrophenylhydrazine in a solution which contains methanol and some concentrated sulfuric acid and the medium should be slightly acidic in nature.
Fractional distillation is the process in which separation of a mixture into its components parts. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. When a mixture of benzaldehyde and formaldehyde is heated with 2,4DNP then two types of organic products will be obtained after fractional distillation which can be shown as:
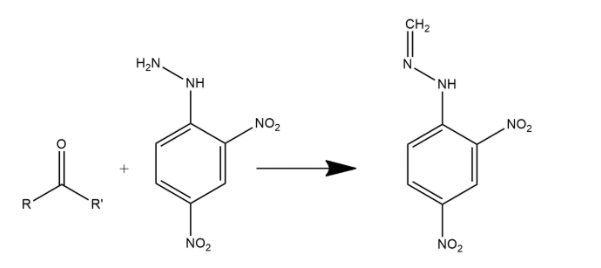
The other product is:
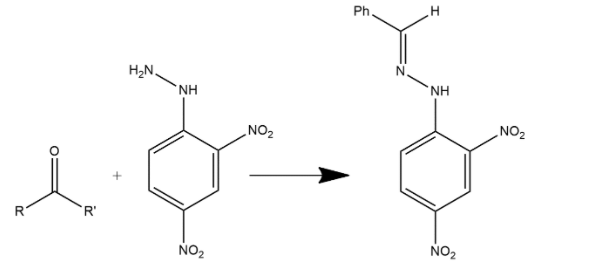
These other two products are very mirror exam products and an even mirror hydrazine coupling products.
Note: 2,4 DNP is generally used to detect the carbonyl functionality of ketone or aldehyde groups. Formation of a yellow, orange or red precipitate gives the signals of positivity of the test and if the carbonyl compound is aromatic then precipitate will be red and if aliphatic then precipitate will be of yellow color.
Complete step by step answer: DNP is generally used as a test to identify the carbonyl group which is associated with aldehyde or ketone. It is generally available as a wet powder which is prepared by the reaction of hydrazine sulfate with 2,4-dinitrochlorobenzene. It is prepared by dissolving 2,4-dinitrophenylhydrazine in a solution which contains methanol and some concentrated sulfuric acid and the medium should be slightly acidic in nature.
Fractional distillation is the process in which separation of a mixture into its components parts. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. When a mixture of benzaldehyde and formaldehyde is heated with 2,4DNP then two types of organic products will be obtained after fractional distillation which can be shown as:

The other product is:

These other two products are very mirror exam products and an even mirror hydrazine coupling products.
Note: 2,4 DNP is generally used to detect the carbonyl functionality of ketone or aldehyde groups. Formation of a yellow, orange or red precipitate gives the signals of positivity of the test and if the carbonyl compound is aromatic then precipitate will be red and if aliphatic then precipitate will be of yellow color.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




