
A compound A ${C_2}{H_6}O$ on oxidation by PCC give B which on treatment with aqueous alkali and subsequent heating gives C. B on oxidation by $KMn{O_4}$ forms a monobasic carboxylic acid with molar mass $60g/mol$ . Identify A,B,C
Answer
575.7k+ views
Hint: PCC is pyridinium chlorochromate. It is a reagent that is used in organic synthesis for the oxidation of alcohol to Carbonyl groups. Carbonyl compounds are aldehydes and ketones. The reagent PCC mentioned in the question oxidizes primary alcohol to aldehydes. Potassium permanganate oxidizes carboxylic acid.
Complete step by step answer:
The given compound is ${C_2}{H_6}O$ which is ethanol ${C_2}{H_5}OH$. Now pyridinium chlorochromate oxidizes it to aldehyde. As it is a primary alcohol, we get the product ethanal. So the product B is ethanal and A is ethanol.
The next step is the treatment of product B ( Ethanal) with Aqueous alkali. We know that an aldehyde on treatment with an aqueous alkali like NaOH undergoes aldol condensation that gives the product $\beta - hydroxy aldehyde$ which on further heating gives $\alpha - \beta - unsaturated{{ }}aldehyde$ (unsaturated Is the presence of double bond).
The reaction is as follows
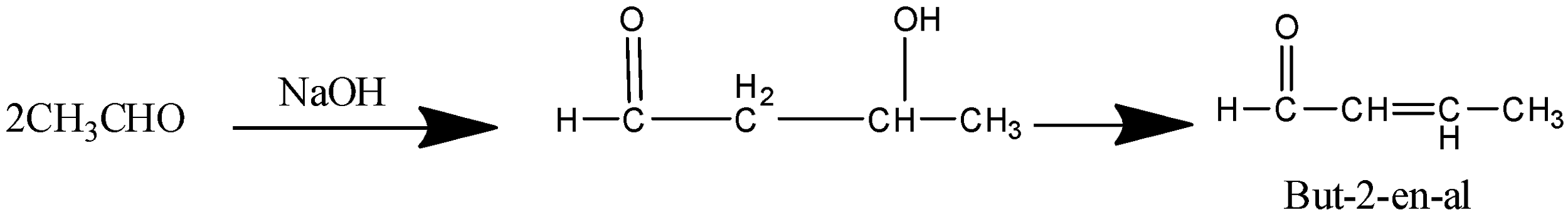
So the product C is $But - 2 - en - al$.
Now it is given that oxidation of B (acetaldehyde) with potassium permanganate gives the corresponding acid. We know that potassium permanganate is a strong oxidizing agent so it converts acetaldehyde into the corresponding acid that is ethanoic acid whose molar mass is $60g/mol$ .
Thus, compounds A, B, C are as follows.
A is Ethanol
B is Ethanal
C is $But - 2 - en - al$
The compound formed on subsequent heating of compound B with potassium permanganate is acetic acid (Ethanoic acid) which is having a molecular mass of sixty.
Note: PCC oxides primary alcohols to aldehydes and secondary alcohols to ketones. Potassium permanganate is a very strong oxidizing agent and it converts almost every compound with oxidizable hydrogen to an acid. The general formula for carboxylic acid is ${C_n}{H_{2n + 1}}COOH$. Here n is the number of carbon atoms. Similarly, the general molecular formula of alcohol is ${C_n}{H_{2n + 1}}OH$.
Complete step by step answer:
The given compound is ${C_2}{H_6}O$ which is ethanol ${C_2}{H_5}OH$. Now pyridinium chlorochromate oxidizes it to aldehyde. As it is a primary alcohol, we get the product ethanal. So the product B is ethanal and A is ethanol.
The next step is the treatment of product B ( Ethanal) with Aqueous alkali. We know that an aldehyde on treatment with an aqueous alkali like NaOH undergoes aldol condensation that gives the product $\beta - hydroxy aldehyde$ which on further heating gives $\alpha - \beta - unsaturated{{ }}aldehyde$ (unsaturated Is the presence of double bond).
The reaction is as follows

So the product C is $But - 2 - en - al$.
Now it is given that oxidation of B (acetaldehyde) with potassium permanganate gives the corresponding acid. We know that potassium permanganate is a strong oxidizing agent so it converts acetaldehyde into the corresponding acid that is ethanoic acid whose molar mass is $60g/mol$ .
Thus, compounds A, B, C are as follows.
A is Ethanol
B is Ethanal
C is $But - 2 - en - al$
The compound formed on subsequent heating of compound B with potassium permanganate is acetic acid (Ethanoic acid) which is having a molecular mass of sixty.
Note: PCC oxides primary alcohols to aldehydes and secondary alcohols to ketones. Potassium permanganate is a very strong oxidizing agent and it converts almost every compound with oxidizable hydrogen to an acid. The general formula for carboxylic acid is ${C_n}{H_{2n + 1}}COOH$. Here n is the number of carbon atoms. Similarly, the general molecular formula of alcohol is ${C_n}{H_{2n + 1}}OH$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




