
A compound used as pistachio flavour in ice cream is
(A) Vanillin
(B) Acetophenone
(C) Muscone
(D) Butyraldehyde
Answer
588.6k+ views
Hint: The compound is a type of aromatic ketone. It is used widely as an active ingredient in food and it imparts fragrance in cherry, strawberry, almond etc. It is also used as a solvent and precursor for resins and plastics.
Complete step by step answer:
- As we know, flavouring reagents are added to food to impart taste or aroma. There are chemical flavours that imitate natural flavours. They are used as additives to enhance the aroma in natural food products which could have got lost due to food processing and to modify the taste.
- The compound that is used as pistachio flavor in ice cream is Acetophenone. It is the organic compound with the formula (${{C}_{6}}{{H}_{5}}COC{{H}_{3}}$). It is the simplest aromatic ketone. Its structure can be given as follows
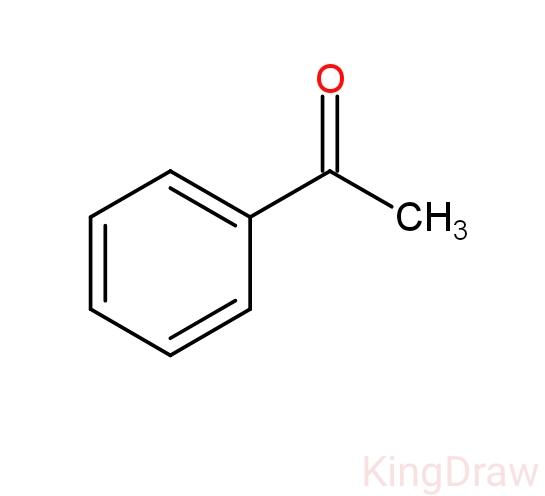
- These aromatic compounds contain a ketone substituted by one alkyl group, and a phenyl group.
- Acetophenone appears as a colorless liquid with a sweet pungent taste and odor resembling the odor of oranges. It is slightly soluble in water and denser than water. Hence sinks in water.
- It freezes under cool conditions. Its vapor is heavier than air. It's a mild irritant to skin and eyes. Vapors can be narcotic in high concentrations
- Acetophenone is a sweet, almond, and acacia tasting compound that can be found in a number of food items such as, wild celery, watermelon, chicory and cloves. Thus, the compound has extensive application as a potential biomarker in the food industry.
-Acetophenone is also used as an intermediate for pharmaceutical, resin and plastic production.
So, the correct answer is “Option B”.
Note: Acetophenone can be recovered as a by-product of the oxidation of ethylbenzene to ethylbenzene hydroperoxide. Acetophenone (${{C}_{6}}{{H}_{5}}COC{{H}_{3}}$) should not be confused with benzophenone
(${{C}_{6}}{{H}_{5}}CO{{C}_{6}}H{{}_{5}}$). They can be distinguished by using iodoform tests. Since the compound benzophenone does not contain any free methyl groups in it, it will give a negative result in iodoform test.
Complete step by step answer:
- As we know, flavouring reagents are added to food to impart taste or aroma. There are chemical flavours that imitate natural flavours. They are used as additives to enhance the aroma in natural food products which could have got lost due to food processing and to modify the taste.
- The compound that is used as pistachio flavor in ice cream is Acetophenone. It is the organic compound with the formula (${{C}_{6}}{{H}_{5}}COC{{H}_{3}}$). It is the simplest aromatic ketone. Its structure can be given as follows

- These aromatic compounds contain a ketone substituted by one alkyl group, and a phenyl group.
- Acetophenone appears as a colorless liquid with a sweet pungent taste and odor resembling the odor of oranges. It is slightly soluble in water and denser than water. Hence sinks in water.
- It freezes under cool conditions. Its vapor is heavier than air. It's a mild irritant to skin and eyes. Vapors can be narcotic in high concentrations
- Acetophenone is a sweet, almond, and acacia tasting compound that can be found in a number of food items such as, wild celery, watermelon, chicory and cloves. Thus, the compound has extensive application as a potential biomarker in the food industry.
-Acetophenone is also used as an intermediate for pharmaceutical, resin and plastic production.
So, the correct answer is “Option B”.
Note: Acetophenone can be recovered as a by-product of the oxidation of ethylbenzene to ethylbenzene hydroperoxide. Acetophenone (${{C}_{6}}{{H}_{5}}COC{{H}_{3}}$) should not be confused with benzophenone
(${{C}_{6}}{{H}_{5}}CO{{C}_{6}}H{{}_{5}}$). They can be distinguished by using iodoform tests. Since the compound benzophenone does not contain any free methyl groups in it, it will give a negative result in iodoform test.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




