
What is a concentration of ores? Explain the process with a diagram by which metallic ore with sulphide as an impurity is purified.
Answer
517.2k+ views
Hint: We know that minerals are present in ores. The solid materials from which a pure metal can be obtained is called ore. The minerals in the ore are present with unwanted minerals called impurities. Froth is produced along with the light particles of the sulphide ore and comes to the surface of the liquid mixture. Heavy particles become wet and settle down at the bottom.
Complete step-by-step solution:
As we know that minerals are present in ores. To extract minerals we have to dig the ore of that mineral. Minerals are present with some unwanted materials called impurities. To remove these unwanted substances the ores are concentrated on the basis of the type of impurities and their percentage proportion.
There are different procedures for the concentration of ores such as hydraulic washing, magnetic separation, and froth floatation refers to separating mineral particles according to the difference in their physical and chemical properties. - This method is particularly used for sulphide minerals as sulphide minerals have high float stability.
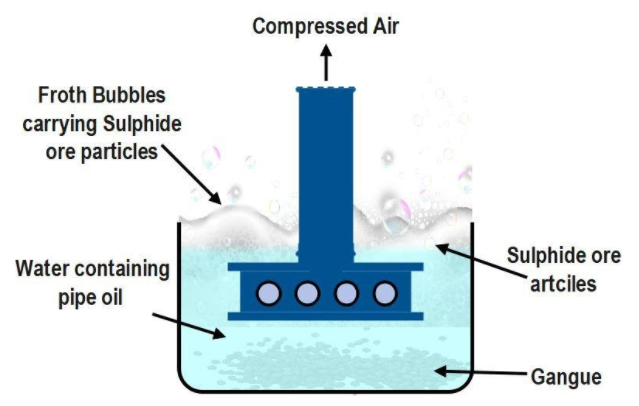
The ores are concentrated on the basis of the type of impurities and their percentage proportions. As a result, most of the impurities are removed and the proportion of the ore is increased. This process is called concentration of the ore. For this,
i) concentration or centrifugation on the basis of difference in densities.
ii) froth floatation and
iii) magnetic separation methods are used.
Froth floatation method is used for concentration of the ores of the metals whose ores are in sulphide form. The concentration of sulphide ores of copper, lead and zinc metal are carried out by this method. In this method, the fine powder of the ore and water are filled in a big vessel. Substances like pine or turpentine oil are added to it.
The sulphide particles of the metals get wet and stick to it, while clay, particles of sand, do not get wet. In this liquid mixture, air is passed with pressure through a tube. Hence, the froth is produced around the light particles of the liquid mixture. Heavy particles like clay, sand etc. become wet by water and settle down at the bottom. The sulphide ore of metal is removed with sieves in a second vessel and washed with water. By this method, ores like copper pyrites are concentrated and clay, sand etc. are removed.
Note: Remember that the process of froth floatation is mainly used for sulphide ores of copper, lead and zinc. Apart from these it can also be used for the concentration of Cinnabar ($HgS$) the ore of mercury. Now the sulphide ore is removed with sieves (a utensil used for straining solid from liquid ) in another vessel and washed with water.
Complete step-by-step solution:
As we know that minerals are present in ores. To extract minerals we have to dig the ore of that mineral. Minerals are present with some unwanted materials called impurities. To remove these unwanted substances the ores are concentrated on the basis of the type of impurities and their percentage proportion.
There are different procedures for the concentration of ores such as hydraulic washing, magnetic separation, and froth floatation refers to separating mineral particles according to the difference in their physical and chemical properties. - This method is particularly used for sulphide minerals as sulphide minerals have high float stability.

The ores are concentrated on the basis of the type of impurities and their percentage proportions. As a result, most of the impurities are removed and the proportion of the ore is increased. This process is called concentration of the ore. For this,
i) concentration or centrifugation on the basis of difference in densities.
ii) froth floatation and
iii) magnetic separation methods are used.
Froth floatation method is used for concentration of the ores of the metals whose ores are in sulphide form. The concentration of sulphide ores of copper, lead and zinc metal are carried out by this method. In this method, the fine powder of the ore and water are filled in a big vessel. Substances like pine or turpentine oil are added to it.
The sulphide particles of the metals get wet and stick to it, while clay, particles of sand, do not get wet. In this liquid mixture, air is passed with pressure through a tube. Hence, the froth is produced around the light particles of the liquid mixture. Heavy particles like clay, sand etc. become wet by water and settle down at the bottom. The sulphide ore of metal is removed with sieves in a second vessel and washed with water. By this method, ores like copper pyrites are concentrated and clay, sand etc. are removed.
Note: Remember that the process of froth floatation is mainly used for sulphide ores of copper, lead and zinc. Apart from these it can also be used for the concentration of Cinnabar ($HgS$) the ore of mercury. Now the sulphide ore is removed with sieves (a utensil used for straining solid from liquid ) in another vessel and washed with water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




