
A) Differentiate between thermoplastics and thermosetting plastics with an example each.
B) Write the monomer unit of Teflon and write anyone's use of Teflon?
Answer
585.3k+ views
Hint: For (A): Thermoplastic polymers are weaker and attached by weak van der Waal forces of attraction while thermosetting polymers are stronger and attached by strong H-bonding.
For (B): Teflon is also known as polytetrafluoroethylene and is formed by the polymerisation of a single monomer.
Complete step by step answer:
A) The polymers differ on the basis of their mechanical properties like toughness, elasticity, tensile strength, etc. These mechanical properties depend on their intermolecular forces like hydrogen bonds and van der Waals forces of attraction which bind the polymer chains together. Depending on these intermolecular forces polymers are divided into 4 types:
(1) Fibres
(2) Elastomers
(3) Thermoplastic polymers
(4) Thermosetting polymers
-Now let’s differentiate between thermoplastic and thermosetting plastics.
B) Teflon:
-Teflon is also known as polytetrafluoroethylene and it is a vinyl polymer.
-It is formed by free radical polymerisation of tetrafluoroethylene. Tetrafluoroethylene is heated with a free radical or persulphate catalyst at high pressures to form Teflon.
The net reaction for its formation is written as:
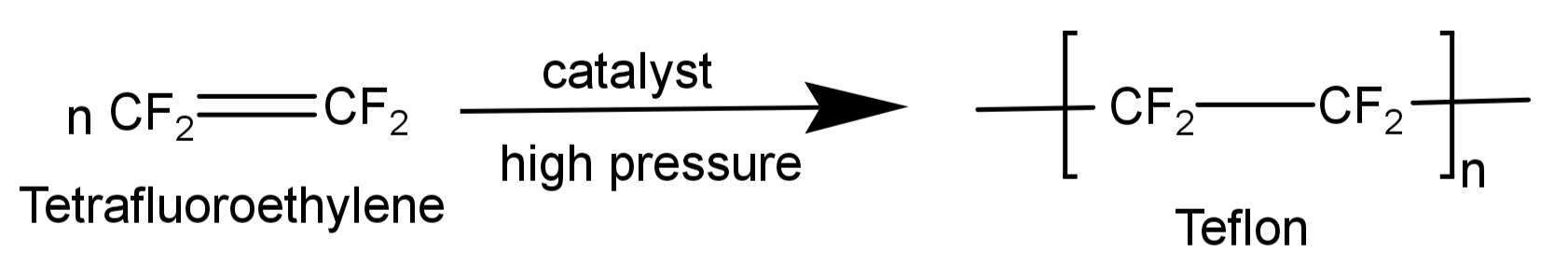
-Hence its monomer is tetrafluoroethylene. Its structure is: $C{F_2} = C{F_2}$
-This polymer is inert chemically and is also resistant to attack by corrosive reagents. It has a melting point of 600 K, high strength, toughness and self lubrication at low temperatures. Teflon has these properties due to the carbon-fluorine (C-F) bonds. It gets depolymerised at temperatures above $650 - {700^ \circ }C$ .
-Uses of Teflon are: in making oil seals, gaskets, non-stick surface coated utensils, production of carbon fibre composites, expansion joints, etc.
Note: For (A): Since the thermosetting polymers are cross linked or 3D structures they are more resistant to any sort of temperature changes, while the thermoplastic polymers are linear in shape and so do not show resistance to temperature variations. For (B): Although Teflon is inert and stable, at temperatures above ${300^ \circ }C$ the Teflon coatings present on the non-stick cookware starts breaking down and toxic chemicals are released which on inhalation may cause polymer fume fever or Teflon fever.
For (B): Teflon is also known as polytetrafluoroethylene and is formed by the polymerisation of a single monomer.
Complete step by step answer:
A) The polymers differ on the basis of their mechanical properties like toughness, elasticity, tensile strength, etc. These mechanical properties depend on their intermolecular forces like hydrogen bonds and van der Waals forces of attraction which bind the polymer chains together. Depending on these intermolecular forces polymers are divided into 4 types:
(1) Fibres
(2) Elastomers
(3) Thermoplastic polymers
(4) Thermosetting polymers
-Now let’s differentiate between thermoplastic and thermosetting plastics.
| Thermoplastic plastics | Thermosetting plastics |
| 1) Such polymers or plastics are usually formed by addition polymerisation. | 1) These plastics are usually formed by condensation polymerisation. |
| 2) They are linear or slightly branched long chain polymers. | 2) They are cross linked or heavily branched. |
| 3) They can be easily soften on heating and hardened on cooling. | 3) It cannot be softened on heating. |
| 4) They are held together by Van der Waal forces of attraction. | 4) They are held together by strong hydrogen bonds. |
| 5) By nature they are soft, weak and less brittle. | 5) They are strong, hard and more brittle in nature. |
| 6) Their molecular weight is low. | 6) Their molecular weight is large. |
| 7) They are quite soluble in organic solvents. | 7) They are insoluble in organic solvents. |
| 8) They can be remoulded into desired shapes. | 8) They cannot be remoulded. |
| 9) Monomers used here do not have more than two reaction sites. | 9) Monomers used here have more than two reaction sites. |
| 10) Examples: polythene, polystyrene, polyvinyls, etc. | 10) Examples: bakelite, urea-formaldehyde resins, etc. |
B) Teflon:
-Teflon is also known as polytetrafluoroethylene and it is a vinyl polymer.
-It is formed by free radical polymerisation of tetrafluoroethylene. Tetrafluoroethylene is heated with a free radical or persulphate catalyst at high pressures to form Teflon.
The net reaction for its formation is written as:

-Hence its monomer is tetrafluoroethylene. Its structure is: $C{F_2} = C{F_2}$
-This polymer is inert chemically and is also resistant to attack by corrosive reagents. It has a melting point of 600 K, high strength, toughness and self lubrication at low temperatures. Teflon has these properties due to the carbon-fluorine (C-F) bonds. It gets depolymerised at temperatures above $650 - {700^ \circ }C$ .
-Uses of Teflon are: in making oil seals, gaskets, non-stick surface coated utensils, production of carbon fibre composites, expansion joints, etc.
Note: For (A): Since the thermosetting polymers are cross linked or 3D structures they are more resistant to any sort of temperature changes, while the thermoplastic polymers are linear in shape and so do not show resistance to temperature variations. For (B): Although Teflon is inert and stable, at temperatures above ${300^ \circ }C$ the Teflon coatings present on the non-stick cookware starts breaking down and toxic chemicals are released which on inhalation may cause polymer fume fever or Teflon fever.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




