
A. How benzene is converted to toluene in two steps?
B. Write the following alkyl halides in the increasing order of their reactivity towards ${{S}_{N}}1$ reaction with reason: ${{\left( C{{H}_{3}} \right)}_{3}}C-F,\text{ }{{\left( C{{H}_{3}} \right)}_{3}}C-Cl,\text{ }{{\left( C{{H}_{3}} \right)}_{3}}C-Br,\text{ }{{\left( C{{H}_{3}} \right)}_{3}}C-I$
Answer
581.7k+ views
Hint: A. To solve this, use Friedel-Crafts alkylation method using a Lewis acid and an alkyl halide. This will be a two-step reaction.
B. Remember that reactivity towards ${{S}_{N}}1$ reaction depends upon stability of the carbocation and also the rate of leaving of the leaving groups. Here, you need to consider the second factor.
Complete step by step solution:
A. We know that benzene is an aromatic organic compound and toluene is an aromatic hydrocarbon. There are several methods to convert benzene to toluene but here we have been asked about the method which involves only two steps. So, let’s discuss the reaction.
In the Friedel-Crafts alkylation, we treat benzene with chloromethane and a Lewis acid like $AlC{{l}_{3}}$.
At first, electrophile i.e. the carbocation is formed from chloromethane and aluminium chloride. Then benzene participates in electrophilic substitution and a cation is formed. Then the hydrogen is removed by the aluminium chloride anion. We can write the reaction as-
\[\begin{align}
& FORMATION\text{ OF ELECTROPHILE} \\
& C{{H}_{3}}Cl+AlC{{l}_{3}}\to C{{H}_{3}}^{+}+AlC{{l}_{4}}^{-} \\
\end{align}\]
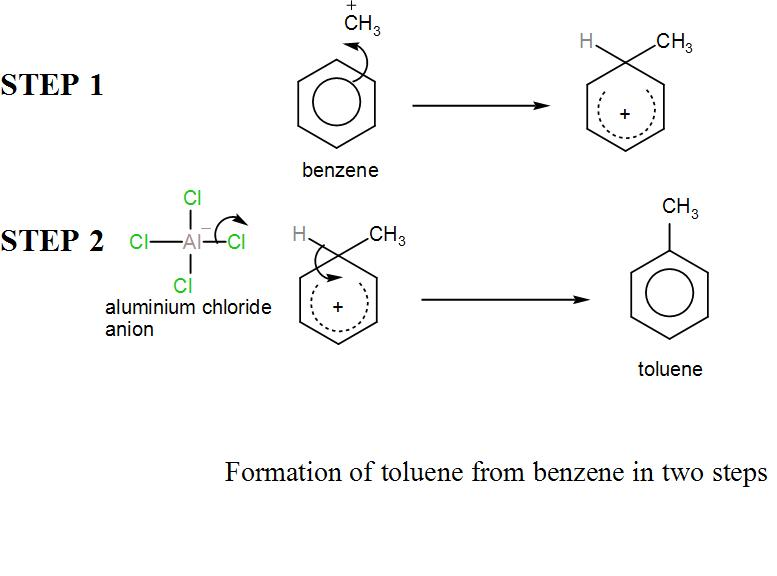
The above reaction is the required answer.
-B. Here, we have to find the rate of reaction of tertiary halides in ${{S}_{N}}1$ reaction. So, firstly discuss the ${{S}_{N}}1$ reaction mechanism. We know that ${{S}_{N}}1$ mechanism is nucleophilic substitution unimolecular reaction. In the first step of the mechanism, the leaving group leaves the substrate and this leads to the formation of a carbocation. Then, the nucleophile attacks the carbocation and gives us the product. This is a two-step process. The reactivity of compounds undergoing ${{S}_{N}}1$ depends upon 2 factors. How effectively the leaving group leaves and whether or not the carbocation formed is stable. Now, let us see the question given to us. Here, we have tertiary halide. The carbocation formed in each case is a tertiary carbocation so we cannot determine the reactivity based upon the stability of carbocation so we’ve to compare which halide is the best leaving group. Now, among the halides, we know that iodine has the largest atomic radii and it is a better nucleophile. This makes iodine a better leaving group. Also, fluorine being the smallest in size is highly electronegative thus breaking the C-F bond is more difficult than breaking a C-I bond.
Therefore, the increasing order of their reactivity towards ${{S}_{N}}1$ reaction will be: ${{\left( C{{H}_{3}} \right)}_{3}}C-F$ < $\text{ }{{\left( C{{H}_{3}} \right)}_{3}}C-Cl$ < ${{\left( C{{H}_{3}} \right)}_{3}}C-Br$ < ${{\left( C{{H}_{3}} \right)}_{3}}C-I$ and this is the required answer.
Note: Similar to the Friedel-Crafts Alkylation, there is another method named Friedel-Crafts Acylation which gives us aryl ketones. The acylation is a better option compared to alkylation because it does not require any particular structural feature in the acyl chloride. Tertiary carbocation are more reactive towards substitution nucleophilic unimolecular whereas primary carbocation are more reactive towards nucleophilic substitution bimolecular.
B. Remember that reactivity towards ${{S}_{N}}1$ reaction depends upon stability of the carbocation and also the rate of leaving of the leaving groups. Here, you need to consider the second factor.
Complete step by step solution:
A. We know that benzene is an aromatic organic compound and toluene is an aromatic hydrocarbon. There are several methods to convert benzene to toluene but here we have been asked about the method which involves only two steps. So, let’s discuss the reaction.
In the Friedel-Crafts alkylation, we treat benzene with chloromethane and a Lewis acid like $AlC{{l}_{3}}$.
At first, electrophile i.e. the carbocation is formed from chloromethane and aluminium chloride. Then benzene participates in electrophilic substitution and a cation is formed. Then the hydrogen is removed by the aluminium chloride anion. We can write the reaction as-
\[\begin{align}
& FORMATION\text{ OF ELECTROPHILE} \\
& C{{H}_{3}}Cl+AlC{{l}_{3}}\to C{{H}_{3}}^{+}+AlC{{l}_{4}}^{-} \\
\end{align}\]

The above reaction is the required answer.
-B. Here, we have to find the rate of reaction of tertiary halides in ${{S}_{N}}1$ reaction. So, firstly discuss the ${{S}_{N}}1$ reaction mechanism. We know that ${{S}_{N}}1$ mechanism is nucleophilic substitution unimolecular reaction. In the first step of the mechanism, the leaving group leaves the substrate and this leads to the formation of a carbocation. Then, the nucleophile attacks the carbocation and gives us the product. This is a two-step process. The reactivity of compounds undergoing ${{S}_{N}}1$ depends upon 2 factors. How effectively the leaving group leaves and whether or not the carbocation formed is stable. Now, let us see the question given to us. Here, we have tertiary halide. The carbocation formed in each case is a tertiary carbocation so we cannot determine the reactivity based upon the stability of carbocation so we’ve to compare which halide is the best leaving group. Now, among the halides, we know that iodine has the largest atomic radii and it is a better nucleophile. This makes iodine a better leaving group. Also, fluorine being the smallest in size is highly electronegative thus breaking the C-F bond is more difficult than breaking a C-I bond.
Therefore, the increasing order of their reactivity towards ${{S}_{N}}1$ reaction will be: ${{\left( C{{H}_{3}} \right)}_{3}}C-F$ < $\text{ }{{\left( C{{H}_{3}} \right)}_{3}}C-Cl$ < ${{\left( C{{H}_{3}} \right)}_{3}}C-Br$ < ${{\left( C{{H}_{3}} \right)}_{3}}C-I$ and this is the required answer.
Note: Similar to the Friedel-Crafts Alkylation, there is another method named Friedel-Crafts Acylation which gives us aryl ketones. The acylation is a better option compared to alkylation because it does not require any particular structural feature in the acyl chloride. Tertiary carbocation are more reactive towards substitution nucleophilic unimolecular whereas primary carbocation are more reactive towards nucleophilic substitution bimolecular.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




