
A hydrocarbon has the molar mass \[86\].The number of chain isomers possible for the compound is
A.five
B.six
C.four
D.ten
Answer
570.6k+ views
Hint: From the given question we know that molar mass of a hydrocarbon is \[86\], we know that the general formula of alkanes is ${C_n}{H_{2n}} + 2$ so we will assume that maximum number of carbon should be $6$ and the remaining will be its hydrogen. We know that if the carbon chain will be more than \[6\] the molecular mass will be greater than \[86\].
Complete step by step answer:
As we know from the hint the hydrocarbon has maximum of $6$ number of carbon so I am writing chemical formula for it which is ${C_6}{H_{14}}$
Checking with molecular formula $12 \times 6'C' + 14 \times 1'H' = 86$
Hence satisfy given molecular formula according to question and next step is chain isomerism
Chain isomers are molecules with the same molecular formula, but different arrangements of the carbon structure.
Now I’m drawing possibilities of chain isomerism of ${C_6}{H_{14}}$
Firstly I’m taking simple structure with straight chain
$CH_3 -CH_2-CH_2-CH_2-CH_2-CH_3$
Hexane
Now just adding $C{H_3}$group on the second carbon chain which is different from above structure.

2 - $Methylpentane$
Adding two $C{H_3}$ group on second carbon which is different from above two diagram
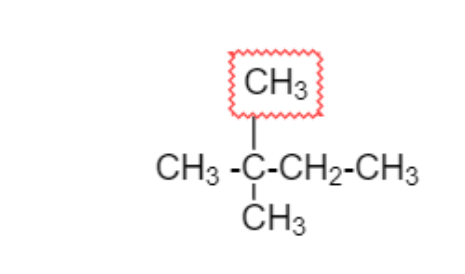
$2,2 - $Dimethylbutane
Here in below diagram straight chain is $C{H_3} - C{H_2} - C{H_2} - C{H_3}$in this straight chain
I’m adding ${C_2}{H_5}$to second carbon and third carbon which is different from above
diagram
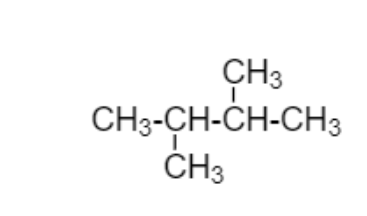
$2,3 - $Dimethylbutane
In the below diagram straight chain is $C{H_3} - C{H_2} - C - C{H_3}$AND I’m adding $C{H_3}$on third carbon atom .
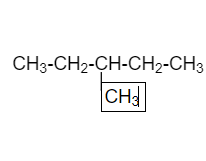
$3 - $methylbutane
Shown images are the number of possible chain isomerism so they are $5$isomerism structure.
Hence answer to this question is option ”A”.
Additional information:
A compound of hydrogen and carbon, like any of these which are the chief components of petroleum and gas
Chain isomerism
It is also referred to as skeletal isomerism.
The components of those isomers display differently branched structures.
Commonly, chain isomers differ within the branching of carbon
Scientists have tried to mathematically derive the amount of isomers of straight-chain organic molecules, called alkanes, but have discovered no simple relationships between isomer count and carbon content. However, computer programs that decompose alkane structures into manageable fragments give good results about alkanes:
Alkanes are chains of carbon (\[C\]) and hydrogen (\[H\]) atoms. For every n carbon atom there are \[\left ({2n {{ }} + {{ }} 2} \right)\] hydrogen atoms. Alkanes originate principally from natural gas and crude oil. The carbon in alkanes forms chains that bind carbon to four other atoms through either \[C - C\] or \[C - H\] bonds. Straight (acyclic) alkanes don't form ring structures. The simplest alkane is methane (\[C {H_4}\]). Alkanes with four or more carbon atoms can form structural isomers, and those with seven or more carbons can also form optical isomers.
Note:The above given structures are chain isomers to each other and if we want to draw any structure they will look the same like this five structure. It's mathematically impossible to calculate the number of isomers of alkanes, but computer programs use an algorithm to work it out.
Complete step by step answer:
As we know from the hint the hydrocarbon has maximum of $6$ number of carbon so I am writing chemical formula for it which is ${C_6}{H_{14}}$
Checking with molecular formula $12 \times 6'C' + 14 \times 1'H' = 86$
Hence satisfy given molecular formula according to question and next step is chain isomerism
Chain isomers are molecules with the same molecular formula, but different arrangements of the carbon structure.
Now I’m drawing possibilities of chain isomerism of ${C_6}{H_{14}}$
Firstly I’m taking simple structure with straight chain
$CH_3 -CH_2-CH_2-CH_2-CH_2-CH_3$
Hexane
Now just adding $C{H_3}$group on the second carbon chain which is different from above structure.

2 - $Methylpentane$
Adding two $C{H_3}$ group on second carbon which is different from above two diagram

$2,2 - $Dimethylbutane
Here in below diagram straight chain is $C{H_3} - C{H_2} - C{H_2} - C{H_3}$in this straight chain
I’m adding ${C_2}{H_5}$to second carbon and third carbon which is different from above
diagram

$2,3 - $Dimethylbutane
In the below diagram straight chain is $C{H_3} - C{H_2} - C - C{H_3}$AND I’m adding $C{H_3}$on third carbon atom .
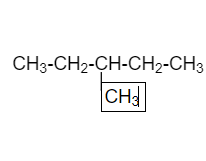
$3 - $methylbutane
Shown images are the number of possible chain isomerism so they are $5$isomerism structure.
Hence answer to this question is option ”A”.
Additional information:
A compound of hydrogen and carbon, like any of these which are the chief components of petroleum and gas
Chain isomerism
It is also referred to as skeletal isomerism.
The components of those isomers display differently branched structures.
Commonly, chain isomers differ within the branching of carbon
Scientists have tried to mathematically derive the amount of isomers of straight-chain organic molecules, called alkanes, but have discovered no simple relationships between isomer count and carbon content. However, computer programs that decompose alkane structures into manageable fragments give good results about alkanes:
Alkanes are chains of carbon (\[C\]) and hydrogen (\[H\]) atoms. For every n carbon atom there are \[\left ({2n {{ }} + {{ }} 2} \right)\] hydrogen atoms. Alkanes originate principally from natural gas and crude oil. The carbon in alkanes forms chains that bind carbon to four other atoms through either \[C - C\] or \[C - H\] bonds. Straight (acyclic) alkanes don't form ring structures. The simplest alkane is methane (\[C {H_4}\]). Alkanes with four or more carbon atoms can form structural isomers, and those with seven or more carbons can also form optical isomers.
Note:The above given structures are chain isomers to each other and if we want to draw any structure they will look the same like this five structure. It's mathematically impossible to calculate the number of isomers of alkanes, but computer programs use an algorithm to work it out.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




