
Why is a metal spoon electroplated?
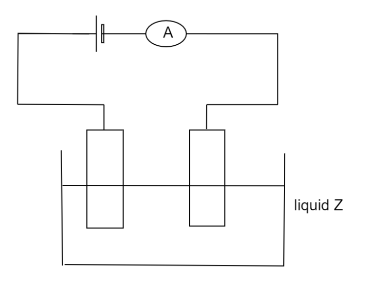
(A) To make the spoon in the desired shape
(B) To prevent corrosion
(C) To prevent the melting of the metal spoon
(D) To make the spoon less expensive

Answer
480.3k+ views
Hint: Electroplating is a chemical process that is done to make the object which is being electroplated highly resistant to heat, look better in appearance, thicker, and resistant to corrosion. Substrates can benefit from the qualities of the metals they are being plated with due to electroplating.
Complete answer:
Electroplating entails sending an electric current via an electrolyte solution. This is accomplished by immersing two electrodes in the electrolyte and connecting them to a circuit powered by a battery or other power source. The electrodes and electrolytes are constructed from carefully selected elements or compounds. When electricity passes through the circuit they create, the electrolyte splits, and some of the metal atoms in it are deposited in a thin layer on top of one of the electrodes, causing it to become electroplated.
By adding a thin, durable metal coating to a surface, electroplating creates a protective barrier that reduces friction and prevents tarnishing while also preserving it from wear and tear. It makes the material resistant to high temperature and also enhances the appearance of the material.
Mainly zinc or tin are used in the electroplating process which is highly corrosion resistant
Therefore the metal spoon is electroplated to prevent corrosion.
Hence option B) to prevent corrosion is the correct option.
Note:
During electroplating a thin layer of the metal is deposited on the spoon so we cannot say it is done to make the spoon in the desired shape. Yes, electroplating does make the melting point of the spoon high but it is not the main reason. Yes, Utensils are gold plated or silver plated to make them less expensive than the real gold and silver utensils. But option B is the correct and appropriate answer.
Complete answer:
Electroplating entails sending an electric current via an electrolyte solution. This is accomplished by immersing two electrodes in the electrolyte and connecting them to a circuit powered by a battery or other power source. The electrodes and electrolytes are constructed from carefully selected elements or compounds. When electricity passes through the circuit they create, the electrolyte splits, and some of the metal atoms in it are deposited in a thin layer on top of one of the electrodes, causing it to become electroplated.
By adding a thin, durable metal coating to a surface, electroplating creates a protective barrier that reduces friction and prevents tarnishing while also preserving it from wear and tear. It makes the material resistant to high temperature and also enhances the appearance of the material.
Mainly zinc or tin are used in the electroplating process which is highly corrosion resistant
Therefore the metal spoon is electroplated to prevent corrosion.
Hence option B) to prevent corrosion is the correct option.
Note:
During electroplating a thin layer of the metal is deposited on the spoon so we cannot say it is done to make the spoon in the desired shape. Yes, electroplating does make the melting point of the spoon high but it is not the main reason. Yes, Utensils are gold plated or silver plated to make them less expensive than the real gold and silver utensils. But option B is the correct and appropriate answer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




