
A mineral having the formula, $A{B_2}$ crystallizes in the ccp lattice with A atoms occupying the lattice points. Select the correct statement(s):
(This question has multiple correct options)
(A) The co-ordination number of A is 8.
(B) The co-ordination number of B is 4.
(C) 100% of the tetrahedral voids are occupied by B atoms.
(D) 50% of the tetrahedral voids are occupied by B atoms.
Answer
521.4k+ views
Hint: The coordination number of any atom is equal to the number of atoms it is in contact with from the surroundings. In a ccp lattice the total number of atoms is 4 (Z) and to find the molecular formula of the entire lattice we multiply Z to the chemical composition of the mineral forming the lattice.
Complete step by step answer:
-First of all we will see what a ccp lattice is. A ccp lattice is similar to a hexagonal close packing since in it the spheres forming the second layer are placed on top of half of the depressions of the first layer. The spheres of the third layer are arranged completely differently from the first two layers and the third layer occupies the octahedral voids of the second layer. The third layer spheres are not in line with the spheres of the first layer. The fourth layer is the same as the first layer and so from here the structure starts repeating. Hence the arrangements of layers are in the order: A – B – C – A – B – C. Also in a ccp lattice all the tetrahedral voids are occupied.
The arrangement of atoms in a ccp lattice looks like:
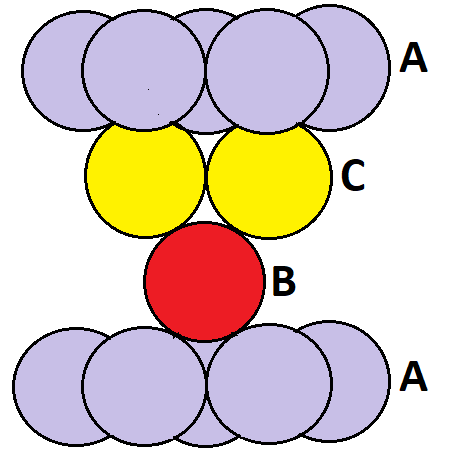
Also for a ccp lattice the packing efficiency is 74%. The total number of atoms present in each ccp lattice is 4 (Z = 4).
-Now coming back to the question, the $A{B_2}$ mineral crystallizes in the ccp lattice and we will now calculate the formula of the entire lattice by multiplying the total number of atoms present in a ccp lattice with the given chemical stoichiometric chemical composition.
Formula of molecule = Z × Chemical composition of mineral
= 4 × $A{B_2}$
= ${A_4}{B_8}$
Hence the molecular formula of the lattice will be: ${A_4}{B_8}$
-We all know that the number of atoms surrounding any given atom will be its coordination number. We will now find out the coordination number of A and B.
From the molecular formula we can tell that A atom is surrounded by 8 atoms of B, hence the coordination number of A will be = 8.
Also we can tell that B atom is surrounded by 4 atoms of A, hence the coordination number of B will be = 4.
-We already know that in a ccp structure all the tetrahedral voids are occupied by the B atoms (as discussed above).
-Hence we can finally say that: the coordination number of A is 8, that of B is 4 and all the tetrahedral voids are filled by the B atoms in the ccp lattice.
So, the correct options will be:
(A) The coordination number of A is 8.
(B) The coordination number of B is 4.
(C) 100% of the tetrahedral voids are occupied by B atoms.
Note: The full form of ccp is cubic close packing and it has a coordination number of 12 because its atoms are in contact with 6 atoms of its own layer, 3 atoms of the top layer and 3 atoms of the bottom layer. For any lattice which has ‘n’ number of atoms:
-The number of octahedral voids = n
-The number of tetrahedral voids = 2n
Since for a ccp lattice the total number of atoms = 4. It will have:
-The number of octahedral voids = 4
-The number of tetrahedral voids = 2 × 4 = 8
Complete step by step answer:
-First of all we will see what a ccp lattice is. A ccp lattice is similar to a hexagonal close packing since in it the spheres forming the second layer are placed on top of half of the depressions of the first layer. The spheres of the third layer are arranged completely differently from the first two layers and the third layer occupies the octahedral voids of the second layer. The third layer spheres are not in line with the spheres of the first layer. The fourth layer is the same as the first layer and so from here the structure starts repeating. Hence the arrangements of layers are in the order: A – B – C – A – B – C. Also in a ccp lattice all the tetrahedral voids are occupied.
The arrangement of atoms in a ccp lattice looks like:

Also for a ccp lattice the packing efficiency is 74%. The total number of atoms present in each ccp lattice is 4 (Z = 4).
-Now coming back to the question, the $A{B_2}$ mineral crystallizes in the ccp lattice and we will now calculate the formula of the entire lattice by multiplying the total number of atoms present in a ccp lattice with the given chemical stoichiometric chemical composition.
Formula of molecule = Z × Chemical composition of mineral
= 4 × $A{B_2}$
= ${A_4}{B_8}$
Hence the molecular formula of the lattice will be: ${A_4}{B_8}$
-We all know that the number of atoms surrounding any given atom will be its coordination number. We will now find out the coordination number of A and B.
From the molecular formula we can tell that A atom is surrounded by 8 atoms of B, hence the coordination number of A will be = 8.
Also we can tell that B atom is surrounded by 4 atoms of A, hence the coordination number of B will be = 4.
-We already know that in a ccp structure all the tetrahedral voids are occupied by the B atoms (as discussed above).
-Hence we can finally say that: the coordination number of A is 8, that of B is 4 and all the tetrahedral voids are filled by the B atoms in the ccp lattice.
So, the correct options will be:
(A) The coordination number of A is 8.
(B) The coordination number of B is 4.
(C) 100% of the tetrahedral voids are occupied by B atoms.
Note: The full form of ccp is cubic close packing and it has a coordination number of 12 because its atoms are in contact with 6 atoms of its own layer, 3 atoms of the top layer and 3 atoms of the bottom layer. For any lattice which has ‘n’ number of atoms:
-The number of octahedral voids = n
-The number of tetrahedral voids = 2n
Since for a ccp lattice the total number of atoms = 4. It will have:
-The number of octahedral voids = 4
-The number of tetrahedral voids = 2 × 4 = 8
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




