
When a mixture of benzaldehyde and acetophenone is treated with dilute ${{NaOH}}$ at $293{{K}}$, it forms
A. A. $2,3 - $ phenylpropanal
B. $1,1 - $diphenylpropan $ - 2 - $ one $ - 1 - $ ol
C. $1,3 - $ diphenylprop $ - 2 - $ ene $ - 1 - $ one
D. $1,2 - $ diphenylprop $ - 2 - $ en $ - 1 - $ one
E. $1,3 - $ diphenylprop $ - 2 - $ en $ - 1 - $ al
Answer
524.8k+ views
Hint:Aldol condensation is a property of compounds having carbonyl groups which have $\alpha - $ hydrogen. This reaction is a reversible reaction. The given reaction can be termed as cross-aldol condensation reaction.
Complete step by step solution:
Aldol means the formation of aldehyde and alcohols on the same molecule. When two or more molecules are combined, there is a loss of small molecules like water or alcohol. This involves nucleophilic addition of enolate ions to the carbonyl group.
The given reaction is known as Claisen-Schmidt condensation reaction. In this reaction, addition of ketone enolate to the aldehyde occurs since the ketone enolate has lower energy and it is formed faster.
This reaction produces, generally, $\alpha ,\beta - $ unsaturated ketones. The ${{C - C}}$ bond is formed in aldol condensation. This formation is facilitated by electron withdrawing groups and stopped by electron releasing groups on the carbonyl group of ketones.
The complete chemical equation of this reaction is given below:
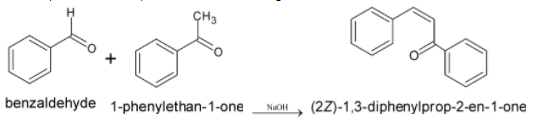
When molecules with $\alpha - $ hydrogen to the carbonyl group are reacted with an alkali, enolate ions are formed. The product formed still has $\alpha - $ hydrogen and a $ - {{OH}}$ group attached to an aromatic ring. Thus it rapidly undergoes condensation reaction by removing water molecules.
Thus the product formed is $1,3 - $ diphenylprop $ - 2 - $ ene $ - 1 - $ one. This compound is also known as benzylideneacetophenone.
Hence, the correct option is C.
Note:
Aldol condensation reaction occurs between two aldehydes or two ketones. When aldol condensation occurs between one aldehyde and one ketone, it is called cross-aldol condensation reaction. The product obtained from the given reaction is also known as chalcone.
Complete step by step solution:
Aldol means the formation of aldehyde and alcohols on the same molecule. When two or more molecules are combined, there is a loss of small molecules like water or alcohol. This involves nucleophilic addition of enolate ions to the carbonyl group.
The given reaction is known as Claisen-Schmidt condensation reaction. In this reaction, addition of ketone enolate to the aldehyde occurs since the ketone enolate has lower energy and it is formed faster.
This reaction produces, generally, $\alpha ,\beta - $ unsaturated ketones. The ${{C - C}}$ bond is formed in aldol condensation. This formation is facilitated by electron withdrawing groups and stopped by electron releasing groups on the carbonyl group of ketones.
The complete chemical equation of this reaction is given below:

When molecules with $\alpha - $ hydrogen to the carbonyl group are reacted with an alkali, enolate ions are formed. The product formed still has $\alpha - $ hydrogen and a $ - {{OH}}$ group attached to an aromatic ring. Thus it rapidly undergoes condensation reaction by removing water molecules.
Thus the product formed is $1,3 - $ diphenylprop $ - 2 - $ ene $ - 1 - $ one. This compound is also known as benzylideneacetophenone.
Hence, the correct option is C.
Note:
Aldol condensation reaction occurs between two aldehydes or two ketones. When aldol condensation occurs between one aldehyde and one ketone, it is called cross-aldol condensation reaction. The product obtained from the given reaction is also known as chalcone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




