
A molecule of ammonia $(N{H_3})$ has:
A. Only single bonds
B. Only double bonds
C. Only triple bonds
D. Two double bonds and one single bond
Answer
527.1k+ views
Hint: Ammonia molecule has \[A{B_3}\] molecular geometry with a lone pair in which the three hydrogen atoms and an unshared pair of electrons are attached to the central nitrogen atom.
Complete step-by-step solution:
Ammonia is a colourless gas, consisting of hydrogen and nitrogen. It has a pungent smell and it is dangerous in its concentrated form. It behaves as a weak base as it forms salts with many acids. Ammonia is also called azane or nitrogen trihydride. It is a polar molecule due to the presence of lone pairs and readily forms hydrogen bonds.
Ammonia is a covalent atom because of the overlap of orbitals of three hydrogen atoms and three \[s{p^3}\] hybrid orbitals of nitrogen in the structure. The fourth \[s{p^3}\] hybrid orbital is denoted by a lone pair. The lone pair exerts an extra repulsion on the three-bonding hydrogen atoms and creates a slight compression to a ${107.3^\circ }$ bond angle. The H-N-H bond angle is ${107.3^\circ }$ , which results in bond pair-lone pair repulsions that push the N-H bonds inward.
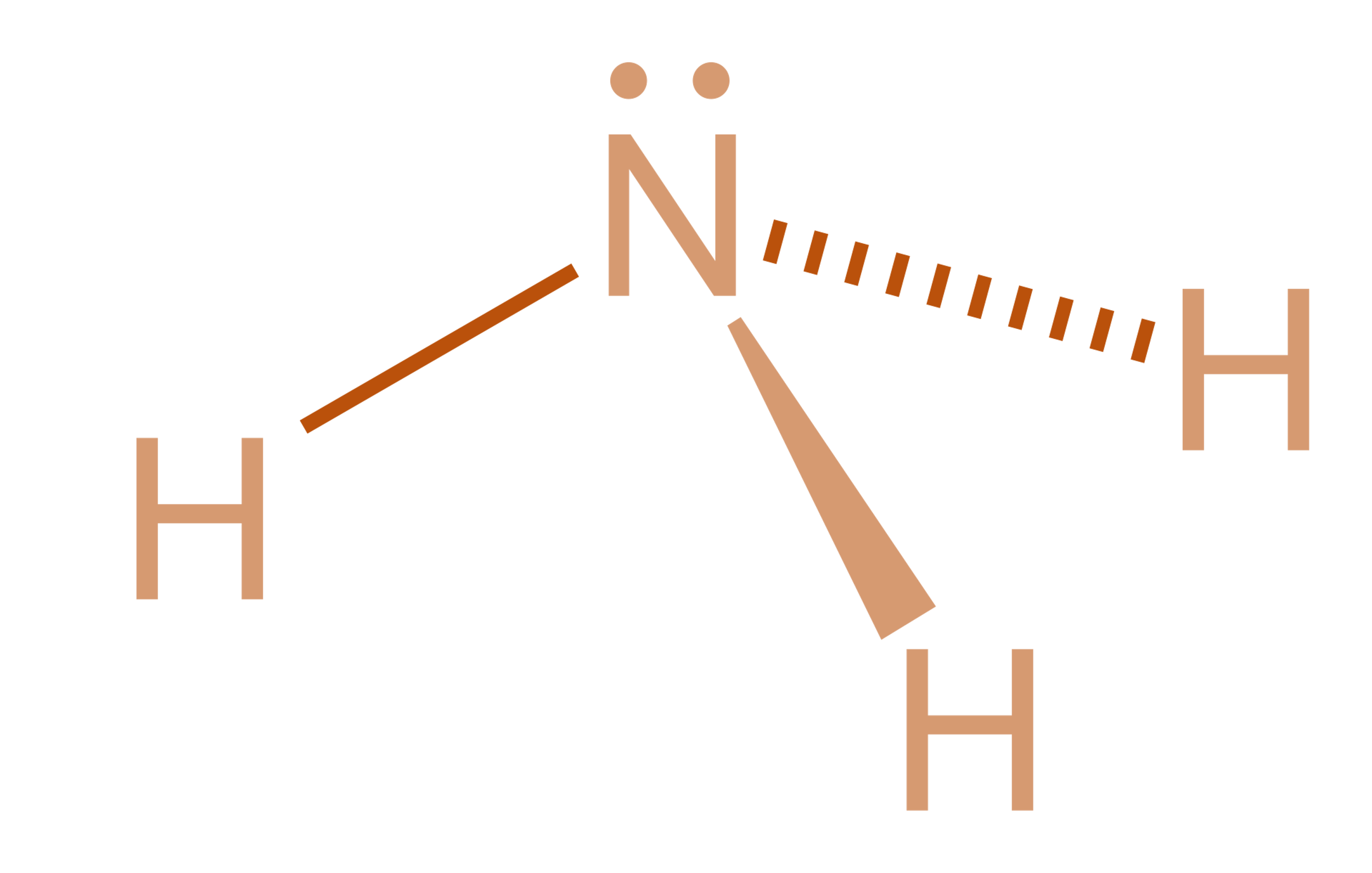
Such a molecule tends to have a trigonal pyramidal molecular geometry because the lone pair although invisible when looking at structure yet exert its influence. Therefore, it has three single bonds and a lone pair.
Hence, the correct option is (A).
Note: The nitrogen has five valence electrons and gets three more electrons from three hydrogen atoms to complete its octet. It leaves a lone electron pair on nitrogen that is not bonded to any other atom and freely available for binding. This might also resemble tetrahedral geometry, having a somewhat similar bond angle of ${109^\circ }$.
Complete step-by-step solution:
Ammonia is a colourless gas, consisting of hydrogen and nitrogen. It has a pungent smell and it is dangerous in its concentrated form. It behaves as a weak base as it forms salts with many acids. Ammonia is also called azane or nitrogen trihydride. It is a polar molecule due to the presence of lone pairs and readily forms hydrogen bonds.
Ammonia is a covalent atom because of the overlap of orbitals of three hydrogen atoms and three \[s{p^3}\] hybrid orbitals of nitrogen in the structure. The fourth \[s{p^3}\] hybrid orbital is denoted by a lone pair. The lone pair exerts an extra repulsion on the three-bonding hydrogen atoms and creates a slight compression to a ${107.3^\circ }$ bond angle. The H-N-H bond angle is ${107.3^\circ }$ , which results in bond pair-lone pair repulsions that push the N-H bonds inward.

Such a molecule tends to have a trigonal pyramidal molecular geometry because the lone pair although invisible when looking at structure yet exert its influence. Therefore, it has three single bonds and a lone pair.
Hence, the correct option is (A).
Note: The nitrogen has five valence electrons and gets three more electrons from three hydrogen atoms to complete its octet. It leaves a lone electron pair on nitrogen that is not bonded to any other atom and freely available for binding. This might also resemble tetrahedral geometry, having a somewhat similar bond angle of ${109^\circ }$.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




