
A photosensitive metal plate is exposed to a monochromatic light beam of frequency which is more than threshold frequency. Then plot \[\dfrac{dN}{dK}\] versus K, is best represented by [N \[=\] Number of photoelectrons, K \[=\] Kinetic energy of photoelectron].
A.
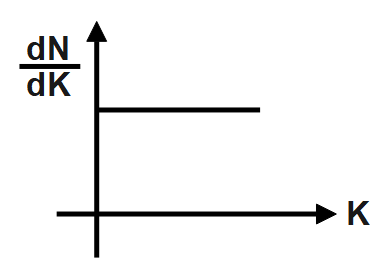
B.
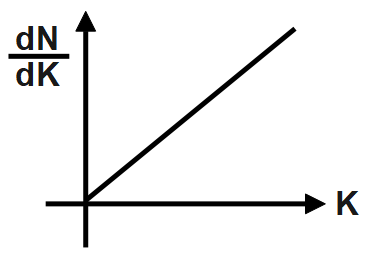
C.
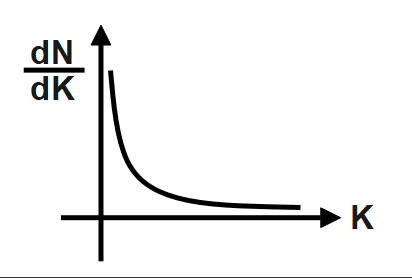
D.
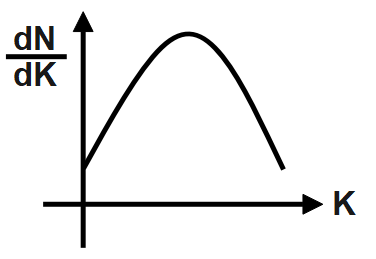




Answer
509.1k+ views
Hint: We know that the kinetic energy of the electron is proportional to the frequency of the incident radiation about a threshold frequency. The threshold frequency for photoelectric emission such that monochromatic light of different frequencies falls on the photosensitive surface, releasing photoelectrons.
Complete answer:
The energy of the photo electrons which are emitted from the photosensitive surface will always remain constant irrespective of the frequency of the monochromatic light rays falling on its surface. According to the photoelectric effect, the total energy of the emitted electrons will be the sum of the fixed potential energy and the variable kinetic energy. Also, according to the photoelectric effect, whenever a light ray falls on the photo-sensitive surface then the photoelectrons get emitted through the surface.
But the light ray should be such that it reaches the threshold energy of the surface and the term threshold frequency is defined as the minimum frequency of the light which causes the electrons to be released from the metal surface when the light falls on it. The threshold frequency is not depending on the intensity of the light. The intensity of the light affects the photoelectric current. The frequency increases the kinetic energy of the photoelectron. Thus, Electromagnetic radiation will release electrons from the solid surface. The photoelectric effect is called this method. Photo emissive is said to be a substance with the photoelectric effect. Photoelectrons are called electrons thrown by the photoelectric effect. Thus, the graph will be linear from both the x and y axis.
Therefore, the correct answer is option B.
Note:
Remember that the photoelectric research resulted in important steps in the realization of light's and electrons' quantum existence and inspired the development of the wave-particle duality principle. Often commonly used to analyze the electron energy level in matter are the photoelectric effects.
Complete answer:
The energy of the photo electrons which are emitted from the photosensitive surface will always remain constant irrespective of the frequency of the monochromatic light rays falling on its surface. According to the photoelectric effect, the total energy of the emitted electrons will be the sum of the fixed potential energy and the variable kinetic energy. Also, according to the photoelectric effect, whenever a light ray falls on the photo-sensitive surface then the photoelectrons get emitted through the surface.
But the light ray should be such that it reaches the threshold energy of the surface and the term threshold frequency is defined as the minimum frequency of the light which causes the electrons to be released from the metal surface when the light falls on it. The threshold frequency is not depending on the intensity of the light. The intensity of the light affects the photoelectric current. The frequency increases the kinetic energy of the photoelectron. Thus, Electromagnetic radiation will release electrons from the solid surface. The photoelectric effect is called this method. Photo emissive is said to be a substance with the photoelectric effect. Photoelectrons are called electrons thrown by the photoelectric effect. Thus, the graph will be linear from both the x and y axis.
Therefore, the correct answer is option B.
Note:
Remember that the photoelectric research resulted in important steps in the realization of light's and electrons' quantum existence and inspired the development of the wave-particle duality principle. Often commonly used to analyze the electron energy level in matter are the photoelectric effects.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




