
What is a reactivity series? How does the reactivity series of metals help in predicting the relative activities of various metals?
Answer
578.1k+ views
Hint:The series in which the elements are arranged in the increasing or decreasing order of their reactivity is known as the Reactivity series of elements. This series is highly useful and is used in the prediction of the reactivity of different elements in order to form the desired products in the reactions.
Complete step by step answer:
The series gives an idea about the fact that which metal will displace the other from its salt solution in the reaction mixture in order to obtain the desired product. The reaction of a particular metal towards the acid and the water can also be predicted by this series. The reactivity series of metals is shown below:
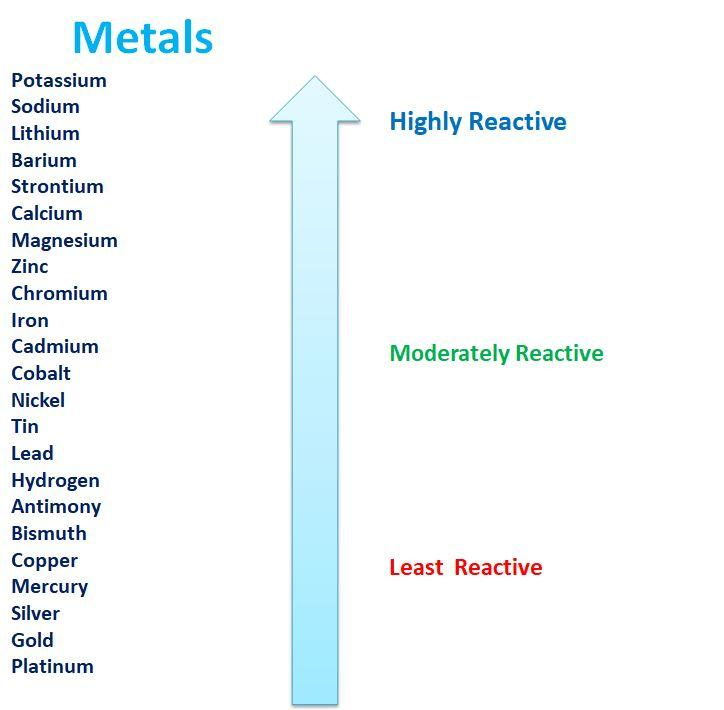
The series given above clearly shows that the metals form the cations readily by losing the electrons. The metal oxides are formed when they react with the atmospheric oxygen. However the reactivities of the different metals towards the oxygen atom vary.
Characteristics of the reactivity series of metals:
1.The metals at the top of the reactivity series are most reactive and they are powerful reducing agents.
2.The reducing ability of the metals decreases down the series since the reactivity of the metals decreases down the series.
3.The electropositive character also decreases down the series.
4.Elements found above the hydrogen in the activity series can liberate Hydrogen during the reaction while the elements below the hydrogen in the reactivity series cannot do so.
5.Metals that are placed higher on the reactivity series can displace the metals placed in the lower portion of the reactivity series however the vice versa is not possible.
Note:
The reactivity series has various important applications, the different reactions and their feasibility can be easily predicted by these metals. The series gives an idea about the reactivity of different metals towards the acids, bases and other metals.
Complete step by step answer:
The series gives an idea about the fact that which metal will displace the other from its salt solution in the reaction mixture in order to obtain the desired product. The reaction of a particular metal towards the acid and the water can also be predicted by this series. The reactivity series of metals is shown below:

The series given above clearly shows that the metals form the cations readily by losing the electrons. The metal oxides are formed when they react with the atmospheric oxygen. However the reactivities of the different metals towards the oxygen atom vary.
Characteristics of the reactivity series of metals:
1.The metals at the top of the reactivity series are most reactive and they are powerful reducing agents.
2.The reducing ability of the metals decreases down the series since the reactivity of the metals decreases down the series.
3.The electropositive character also decreases down the series.
4.Elements found above the hydrogen in the activity series can liberate Hydrogen during the reaction while the elements below the hydrogen in the reactivity series cannot do so.
5.Metals that are placed higher on the reactivity series can displace the metals placed in the lower portion of the reactivity series however the vice versa is not possible.
Note:
The reactivity series has various important applications, the different reactions and their feasibility can be easily predicted by these metals. The series gives an idea about the reactivity of different metals towards the acids, bases and other metals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




