
A solution in which still more number of solute can be dissolved, is known as.
A. unsaturated
B. supersaturated
C. saturated
D. dilute
E. concentrated
Answer
573.9k+ views
Hint: We know that the solution contains many properties like saturation, un-saturation dilution, and supersaturation that can be identified by the number of solute particles dissolved in the solution.
Complete answer
First, we discuss unsaturated, it is specified when the solvent holds an amount of solute less than that amount which would be in equilibrium with undissolved solute. Any solvent can dissolve solute to a certain extent. When at room temperature you dissolve a solute in a solvent it is known as unsaturated solution.
Let's discuss supersaturated, a supersaturated solution is an unstable solution where the concentration of the compounds is greater than the compounds solubility. Well the solubility of the compounds is measured under temperature, pressure co-solvents. Sometimes a solution can be made under non-equilibrium conditions. And may temporarily become supersaturated.
As we know that, a saturated solution is a solution with a solvent that dissolves until it can no longer dissolve, and an undissolved substance appears as a precipitate. When we speak of saturation with respect to solution it is best to speak of an equilibrium condition. Saturated chemicals are very concentrated, and cannot take much more of them.
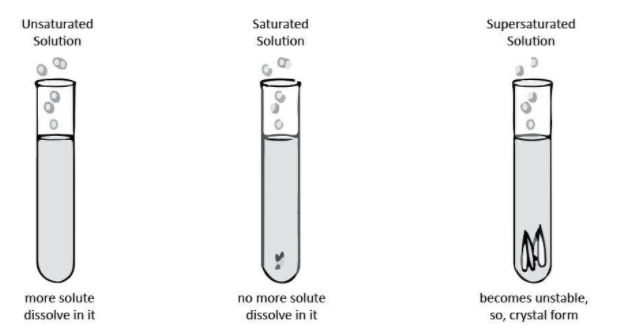
And when we say about the dilution and concentration, it means when some amount of water is added to any solution so it can become dilute from concentrated from its known as dilute solution. Or we can say the amount of water or solvent is more than solute.
Therefore, the correct answer is A.
Note:
The solubility of a compound in a particular solvent is due to physical and chemical properties of the solute and the solvent, ionic polar, ability to make hydrogen bonds etc.
Complete answer
First, we discuss unsaturated, it is specified when the solvent holds an amount of solute less than that amount which would be in equilibrium with undissolved solute. Any solvent can dissolve solute to a certain extent. When at room temperature you dissolve a solute in a solvent it is known as unsaturated solution.
Let's discuss supersaturated, a supersaturated solution is an unstable solution where the concentration of the compounds is greater than the compounds solubility. Well the solubility of the compounds is measured under temperature, pressure co-solvents. Sometimes a solution can be made under non-equilibrium conditions. And may temporarily become supersaturated.
As we know that, a saturated solution is a solution with a solvent that dissolves until it can no longer dissolve, and an undissolved substance appears as a precipitate. When we speak of saturation with respect to solution it is best to speak of an equilibrium condition. Saturated chemicals are very concentrated, and cannot take much more of them.

And when we say about the dilution and concentration, it means when some amount of water is added to any solution so it can become dilute from concentrated from its known as dilute solution. Or we can say the amount of water or solvent is more than solute.
Therefore, the correct answer is A.
Note:
The solubility of a compound in a particular solvent is due to physical and chemical properties of the solute and the solvent, ionic polar, ability to make hydrogen bonds etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




