
A solution of m-chloroaniline, m-chlorophenol and m- chlorobenzoic acid in ethyl acetate was extracted initially with a saturated solution of \[NaHCO_3\] to give fraction \[A\]. The leftover organic phase was extracted with a dilute \[NaOH\] solution to give fraction \[B\]. The final organic layer was labelled as fraction \[C\]. Fractions \[A,B,C\] contain respectively:
A.m-chloroaniline,m-chlorobenzoic acid and m-chlorophenol
B.m-chlorophenol, m-chlorobenzoic acid and m-chloroaniline
C.m-chlorobenzoic acid,m-chlorophenol and m-chloroaniline
D.m-chlorobenzoic acid, m-chloroaniline and m- chlorophenol
Answer
578.7k+ views
Hint: To solve this question accurately we need to understand the structures of all the given compounds that are m-chloroaniline, m-chlorophenol and m-chlorobenzoic acid. Let’s have a look at the structures.

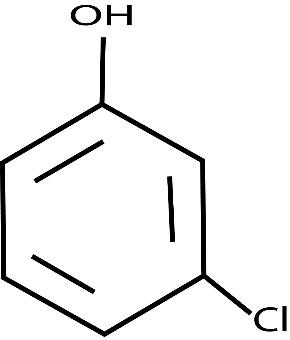
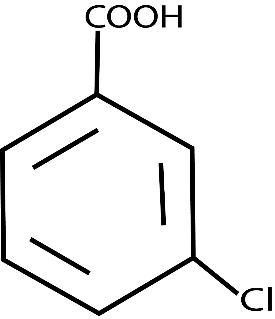
m-CHLOROANILINE m-CHLOROPHENOL m-CHLOROBENZOIC ACID
Also,\[NaHCO_3\] is a weak base. M-chloroaniline we know is base and m-chlorophenol and m-chlorobenzoic acid these two both are acids. But m-chlorobenzoic acid is a strong acid as compared to m-chlorophenol and NaOH is a strong base.Now what the question says is that there is a solution of these three compounds and each compound needs to be extracted one after the other. Fraction \[A\] to be extracted using concentrated \[NaHCO_3\] then fraction \[B\] to be extracted using dilute \[NaOH\] from the remaining organic phase. The final organic compound that will be left is fraction \[C\].
Complete step by step answer:
By now you must have guessed the correct answer. In case you haven’t let me explain it to you. We know that a weak base always reacts with a strong acid to form an acidic solution. A strong base always reacts with a weak acid to give a basic solution. So, here as I already said \[NaHCO_3\] is a weak base which will react ( that is extract the H positive ion ) with a strong acid (here it is m-chlorobenzoic acid) , so fraction A is going to be m-Chlorobenzoic acid. Remaining solution has m-chloroaniline and m-chlorophenol. Next we have a strong dilute base that is \[NaOH\] and a strong base reacts with a weak acid ( which is m-chlorophenol) in this case. So, even fraction \[B\] is extracted which is m- chlorophenol. Now m-chloroaniline is left so the fraction \[C\] is m-chloroaniline.
So, we have deduced that Fraction \[A\] is m-chlorobenzoic acid, Fraction \[B\] is m-chlorophenol and fraction \[C\] is m-chloroaniline.
So, the correct answer is option (C)
Note:
To solve questions like these you should have a proper understanding of the structures of organic compounds, their acidic or basic character . Like carboxylic groups are highly acidic, then comes phenols and then comes alcohols. Another point in case you don’t know, the ‘m’ written in front of the names of compounds represents the position of chlorine that is meta.



m-CHLOROANILINE m-CHLOROPHENOL m-CHLOROBENZOIC ACID
Also,\[NaHCO_3\] is a weak base. M-chloroaniline we know is base and m-chlorophenol and m-chlorobenzoic acid these two both are acids. But m-chlorobenzoic acid is a strong acid as compared to m-chlorophenol and NaOH is a strong base.Now what the question says is that there is a solution of these three compounds and each compound needs to be extracted one after the other. Fraction \[A\] to be extracted using concentrated \[NaHCO_3\] then fraction \[B\] to be extracted using dilute \[NaOH\] from the remaining organic phase. The final organic compound that will be left is fraction \[C\].
Complete step by step answer:
By now you must have guessed the correct answer. In case you haven’t let me explain it to you. We know that a weak base always reacts with a strong acid to form an acidic solution. A strong base always reacts with a weak acid to give a basic solution. So, here as I already said \[NaHCO_3\] is a weak base which will react ( that is extract the H positive ion ) with a strong acid (here it is m-chlorobenzoic acid) , so fraction A is going to be m-Chlorobenzoic acid. Remaining solution has m-chloroaniline and m-chlorophenol. Next we have a strong dilute base that is \[NaOH\] and a strong base reacts with a weak acid ( which is m-chlorophenol) in this case. So, even fraction \[B\] is extracted which is m- chlorophenol. Now m-chloroaniline is left so the fraction \[C\] is m-chloroaniline.
So, we have deduced that Fraction \[A\] is m-chlorobenzoic acid, Fraction \[B\] is m-chlorophenol and fraction \[C\] is m-chloroaniline.
So, the correct answer is option (C)
Note:
To solve questions like these you should have a proper understanding of the structures of organic compounds, their acidic or basic character . Like carboxylic groups are highly acidic, then comes phenols and then comes alcohols. Another point in case you don’t know, the ‘m’ written in front of the names of compounds represents the position of chlorine that is meta.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




